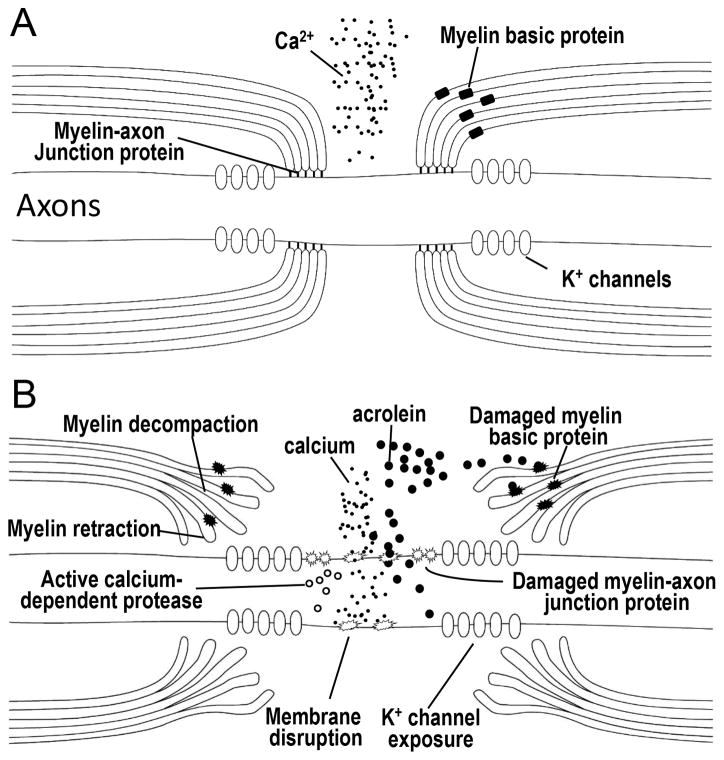
Figure 1.
Diagram representing the steps in acrolein-mediated myelin damage. For healthy nerves (A), proteins such as Caspr, contactin, Nfac, and spectrin comprise axoglial septate junctions that secure the myelin to the axon at the periphery of the nodes of Ranvier. These act to confine voltage gated fast K+ channels to the internode region. Myelin basic protein acts to hold the multi-layer of myelin together, forming a dense and compact structure of myelin. Intact axonal membranes at the nodal region maintain an ionic balance with low concentrations of intracellular Ca2+, keeping intracellular proteases inactive. Exposure to acrolein results in damage to myelin, myelin basic protein, the myelin–axon junction, and the axonal membranes (B). Influx of calcium activates proteases, which may cause further damage to the paranodal axoglial junction which result in detachment of myelin from axon and the retraction of myelin. Damage to the myelin and myelin basic protein lead to myelin decompaction. Ultimately, myelin decompaction and retraction cause exposure of K+ channels and prevent saltatory conduction and consequently result in axonal conduction block and disruption of neuronal function.