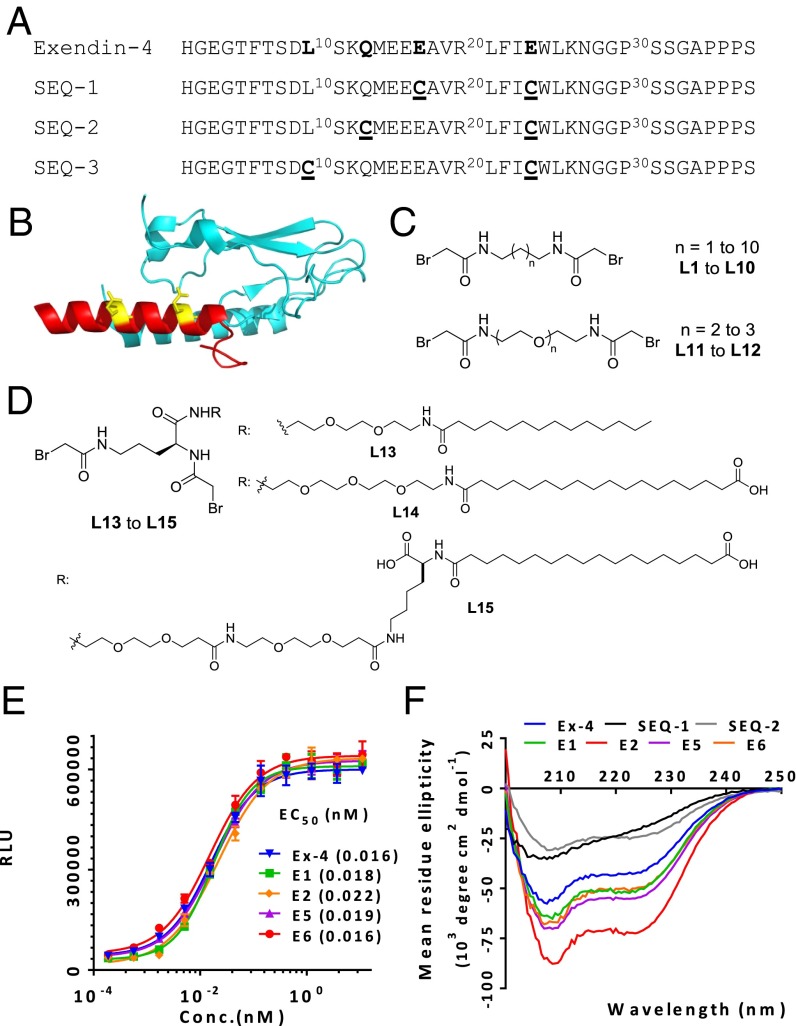
Fig. 1.
Design, in vitro activity, and alpha-helicity of cross-linked Ex-4 analogs. (A) Sequences of Ex-4 and its double cysteine mutants spaced at i, i+7; i, i+11, and i, i+14. (B) Structural model of Ex-4 (9-39) bound to GLP-1 receptor amino-terminal domain (PDB ID code 3C59), with the cross-linking sites colored in yellow. (C) Structures of cross-linkers containing bromoacetamide moiety: alkyl-based L1 to L10, PEG-based L11 to L12, and ornithine-derived PEG-fatty acid–based L13 to L15 linkers (D). (E) In vitro activity of representative cross-linked peptides in the GLP-1R–mediated CRE-Luc reporter assay. (F) CD analysis of cross-linked peptides. Data represent mean ± SEM for experiments performed in triplicate.