Significance
Nigrostriatal dopaminergic projections form a number of dopamine synapses onto medium spiny neurons in the striatum, and have strong influence on emotion, motivation, voluntary movement, and cognition. Despite the functional importance, the molecular composition at dopamine synapses remains unknown. Here we demonstrate that dopamine synapses are neurochemically mismatched contacts formed between dopaminergic presynaptic and GABAergic postsynaptic structures. Intriguingly, neuroligin-2 expressed at the GABAergic postsynaptic structure controls striatal synapse formation by giving competitive advantage to heterologous dopamine synapses over conventional GABAergic synapses. Therefore, our findings suggest that neuroligin-mediated formation of such neurochemically mismatched synapses could be a novel strategy to increase the target selectivity and potency of modulation by anchoring release sites of neuromodulators to their receptive targets.
Keywords: dopamine synapse, neuroligin-2, medium spiny neuron, striatum
Abstract
Midbrain dopamine neurons project densely to the striatum and form so-called dopamine synapses on medium spiny neurons (MSNs), principal neurons in the striatum. Because dopamine receptors are widely expressed away from dopamine synapses, it remains unclear how dopamine synapses are involved in dopaminergic transmission. Here we demonstrate that dopamine synapses are contacts formed between dopaminergic presynaptic and GABAergic postsynaptic structures. The presynaptic structure expressed tyrosine hydroxylase, vesicular monoamine transporter-2, and plasmalemmal dopamine transporter, which are essential for dopamine synthesis, vesicular filling, and recycling, but was below the detection threshold for molecules involving GABA synthesis and vesicular filling or for GABA itself. In contrast, the postsynaptic structure of dopamine synapses expressed GABAergic molecules, including postsynaptic adhesion molecule neuroligin-2, postsynaptic scaffolding molecule gephyrin, and GABAA receptor α1, without any specific clustering of dopamine receptors. Of these, neuroligin-2 promoted presynaptic differentiation in axons of midbrain dopamine neurons and striatal GABAergic neurons in culture. After neuroligin-2 knockdown in the striatum, a significant decrease of dopamine synapses coupled with a reciprocal increase of GABAergic synapses was observed on MSN dendrites. This finding suggests that neuroligin-2 controls striatal synapse formation by giving competitive advantage to heterologous dopamine synapses over conventional GABAergic synapses. Considering that MSN dendrites are preferential targets of dopamine synapses and express high levels of dopamine receptors, dopamine synapse formation may serve to increase the specificity and potency of dopaminergic modulation of striatal outputs by anchoring dopamine release sites to dopamine-sensing targets.
Chemical synapses comprise presynaptic machinery for transmitter release and postsynaptic machinery for receptor-mediated signal transduction in a neurochemically matched manner. They are classified into Gray type-I and type-II synapses by asymmetric or symmetric membrane density, respectively, of the pre- and postsynaptic structures (1). Most, if not all, asymmetric and symmetric synapses are excitatory and inhibitory, respectively, as they selectively express ionotropic glutamate or GABA/glycine receptors together with their specific scaffolding proteins (2). Neurochemical matching of chemical synapses is controlled by activity-dependent mechanisms (3, 4), and mediated by transmembrane adhesion proteins and secreted molecules (5). The neuroligin (NL) family comprises postsynaptic adhesion molecules that form transsynaptic contacts with presynaptic neurexins (Nrxn) (6). Of note, NL1 and NL2 are selectively expressed at glutamatergic and GABAergic synapses, respectively (7, 8), and are required for activity-dependent specification of the corresponding synapses (9).
Midbrain dopamine neurons project densely to the striatum and are strongly involved in motor and cognitive functions (10, 11). Anatomically, dopamine synapses are frequently found on dendritic shafts and spines of GABAergic medium spiny neurons (MSNs), and exhibit ultrastructural features common to symmetric synapses (12–14). MSNs constitute around 90% of all striatal neurons. They are divided equally into direct and indirect pathway MSNs (d-MSN and i-MSN), differing in their connectivity with output nuclei of the basal ganglia and in the expression of dopamine receptors (i.e., D1R in d-MSNs and D2R in i-MSNs) (10). Considering the broad extrasynaptic expression of D1R and D2R (15–17), the mode of dopaminergic transmission at dopamine synapses (i.e., whether it is mediated by wired transmission like that at conventional glutamatergic and GABAergic synapses) remains unclear. It is also unknown which types of molecules comprise pre- and postsynaptic membrane specializations at dopamine synapses.
In the present study, we show that the presynaptic side of dopamine synapses is indeed dopaminergic, whereas the postsynaptic side is exclusively GABAergic, expressing GABAA receptor α1 (GABAARα1), gephyrin, and NL2. In vivo knockdown of NL2 in the striatum reduced the density of dopamine synapses on MSN dendrites, but reciprocally increased that of GABAergic synapses. Our findings suggest that NL2 regulates striatal synapse formation by giving competitive advantage to dopamine synapses over GABAergic synapses, and that the formation of dopamine synapses may serve to provide dopamine-sensing MSNs with structural anchorage of dopamine release sites.
Results
Presynaptic Phenotypes at Dopamine Synapses.
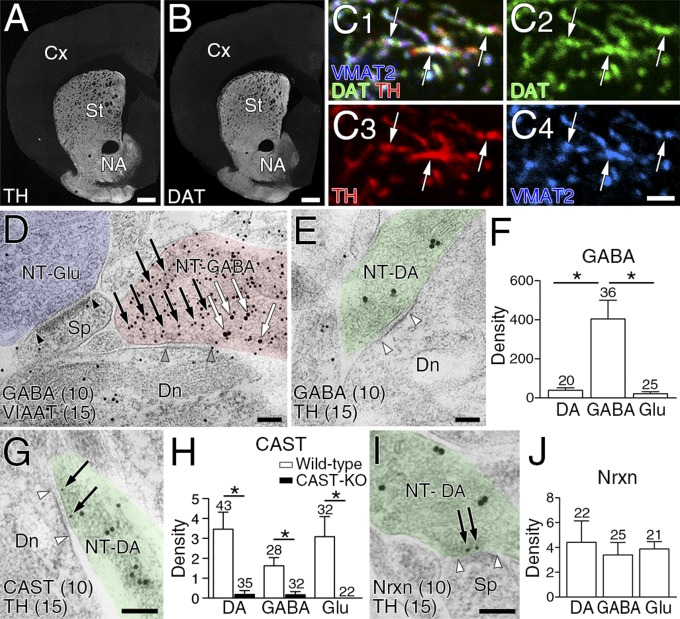
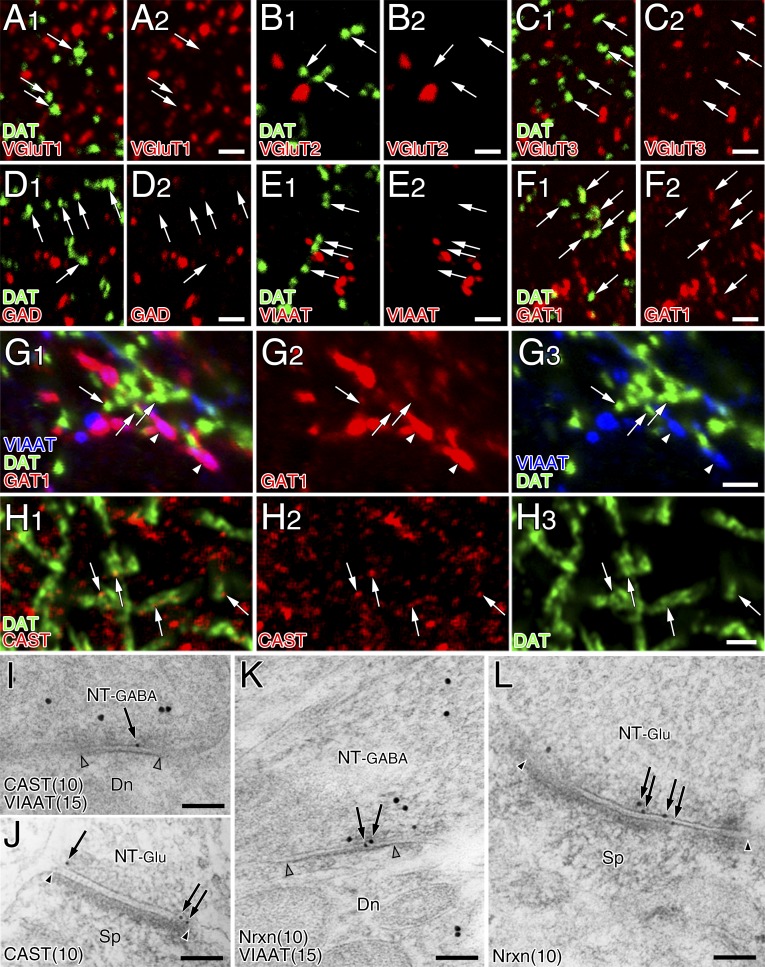
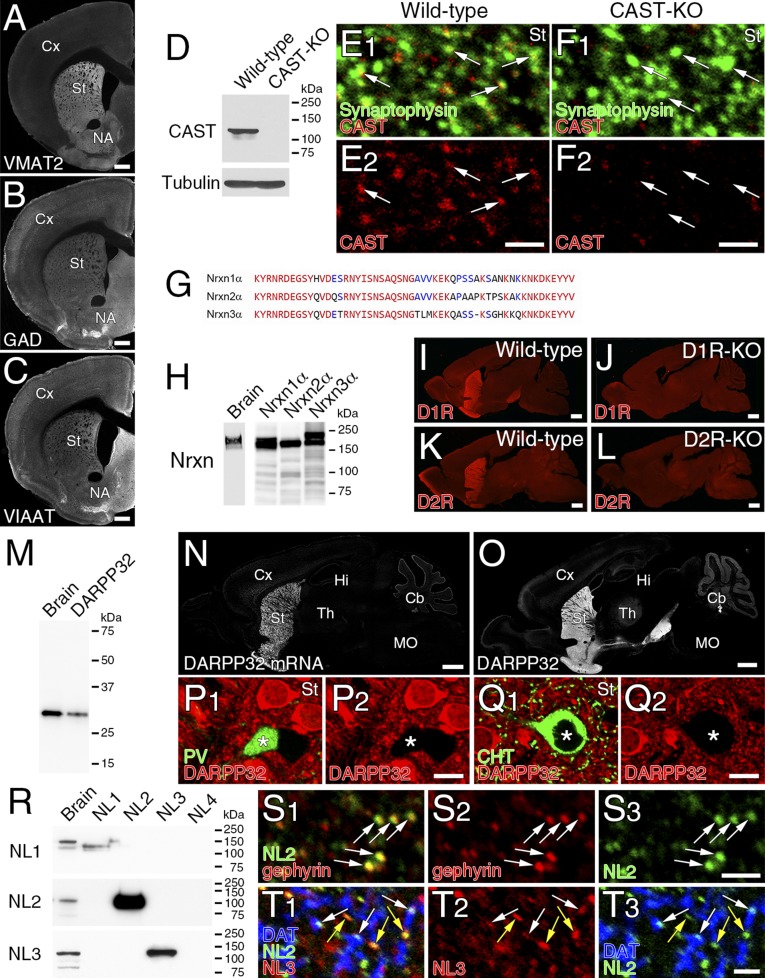
To characterize the molecular–anatomical organization of the so-called dopamine synapse, we studied the dorsolateral striatum in the adult mouse. We first examined the neurochemical properties of dopaminergic terminals, which were identified by immunolabeling for plasmalemmal dopamine transporter (DAT) or tyrosine hydroxylase (TH) (Fig. 1 A and B). Triple immunofluorescence revealed that DAT-labeled dopaminergic terminals overlapped extensively with TH and vesicular monoamine transporter-2 (VMAT2) (Fig. 1C). DAT-labeled dopaminergic terminals had no immunodetectable signals for glutamatergic terminal markers, vesicular glutamate transporters VGluT1, VGluT2, and VGluT3, or for GABAergic terminal markers, 65/67-kDa glutamic acid decarboxylases (GAD) or vesicular inhibitory amino acid transporter (VIAAT) (Fig. S1 A–E). However, plasmalemmal GABA transporter GAT1 was expressed in dopaminergic terminals, albeit at a much lower intensity than in VIAAT-labeled GABAergic terminals (Fig. S1 F and G). We then compared the GABA content in dopaminergic, GABAergic, and glutamatergic terminals using immunoelectron microscopy for GABA and TH or VIAAT. According to previous studies (12, 13, 18), TH- or VIAAT-labeled terminals forming symmetric synapses were judged to be dopaminergic or GABAergic, respectively. Terminals that were unlabeled for either TH or VIAAT and formed asymmetric synapses were judged to be glutamatergic, although a few asymmetric synapses are serotonergic (19). GABAergic terminals were intensely labeled for GABA (Fig. 1D, red), whereas dopaminergic (Fig. 1E, green) and glutamatergic (Fig. 1D, blue) terminals were scarcely labeled for GABA. Density quantification revealed a significantly higher level of immunogold labeling for GABA in GABAergic terminals than in dopaminergic and glutamatergic terminals (Fig. 1F). Therefore, dopaminergic terminals contain very little, if any, GABA and GABAergic proteins, except for GAT1.
Fig. 1.
Dopaminergic presynaptic phenotype at striatal dopamine synapses. (A and B) Immunofluorescence labeling for TH (A) and DAT (B) in the striatum. Cx, cortex; NA, nucleus accumbens; St, striatum. (C) Triple immunofluorescence for TH (red), DAT (green), and VMAT2 (blue) in ultrathin (100 nm) cryosections showing their extensive overlap (arrows). (D and E) Double-label postembedding immunoelectron microscopy for GABA [Ø (diameter) = 10-nm colloidal gold particles] and VIAAT (D, Ø = 15 nm) or TH (E, Ø = 15 nm). GABA (arrows) is concentrated on VIAAT+ GABAergic terminals forming symmetric synapses (NT-GABA, red terminal), but not detected in VIAAT− glutamatergic terminals forming asymmetric synapses (NT-Glu, blue) or TH+ dopaminergic terminals (NT-DA, green) forming symmetric synapses. Arrowhead pairs indicate the synaptic membrane. Dn, dendrite; NT, nerve terminal; Sp, spine. (F) The density of immunogold labeling for GABA (particles per 1 μm2) in dopaminergic (DA), GABAergic (GABA), and glutamatergic (Glu) terminals. (G and I) Double-label postembedding immunogold microscopy for TH (Ø = 15 nm) and CAST (G, Ø = 10 nm) or Nrxn (I, Ø = 10 nm). Immunogold labeling (arrows) for CAST and Nrxn is observed beneath the presynaptic membrane of TH+ dopaminergic terminals. (H and J) The mean density of immunogold particles per 1 μm of synaptic membrane for CAST (H) and Nrxn (J) at dopaminergic, GABAergic, and glutamatergic synapses in wild-type (H and J, open columns) and CAST-KO (H, filled columns) mice. In F, H, and J, numbers of terminals analyzed are indicated above each column, and error bars on columns represent SEM. *P < 0.05 (unpaired t test). [Scale bars: 1 mm (A and B), 2 μm (C), and 100 nm (D, E, G, and I).]
Fig. S1.
Presynaptic phenotype at striatal dopamine synapses is neither glutamatergic nor GABAergic. (A–F) Double immunofluorescence for DAT (green) and terminal markers (red), including VGluT1 (A), VGluT2 (B), VGluT3 (C), GAD (D), VIAAT (E), or GAT1 (F). Ultrathin (100 nm) sections were used to increase the spatial resolution of fluorescent signals. Note the lack of glutamatergic and GABAergic molecular expression in DAT+ dopaminergic terminals, except for weak immunoreactivity for GAT1 (arrows in F). (G) Triple immunofluorescence for DAT (green), GAT1 (red), and VIAAT (blue) showing much stronger expression of GAT1 in VIAAT-labeled GABAergic terminals (arrowheads) than in DAT-labeled dopaminergic terminals (arrows). (H) Superresolution immunofluorescence images for CAST (red) and DAT (green). Tiny CAST+ puncta are detected in some portions of large DAT+ dopaminergic terminals (arrows). (I–L) Double-label postembedding immunoelectron microscopy for VIAAT [Ø (diameter) = 15-nm colloidal gold particles] and CAST (I and J, Ø = 10 nm) or Nrxn (K and L, Ø = 10 nm). Immunogold labeling for CAST and Nrxn is observed beneath the presynaptic membrane at VIAAT+ symmetric or GABAergic synapses (NT-GABA, I and K) and VIAAT− asymmetric or glutamatergic synapses (NT-Glu, J and L). Pairs of open and filled arrowheads indicate the synaptic membrane of symmetric and asymmetric synapses, respectively. Dn, dendrite; Sp, spine. [Scale bars: 2 μm (A–H) and 100 nm (I–L).]
Presynaptic differentiation of dopaminergic terminals was tested by expression of the active zone protein CAST and the presynaptic adhesion molecule Nrxn. Immunofluorescence showed punctate immunolabeling for CAST in DAT+ dopaminergic terminals (Fig. S1H). Immunoelectron microscopy revealed CAST expression beneath the presynaptic membrane of dopaminergic (Fig. 1G), GABAergic (Fig. S1I), and glutamatergic (Fig. S1J) terminals. The density of immunogold labeling for CAST on the presynaptic membrane was comparable across the three types of terminals (Fig. 1H, open columns), and specificity was confirmed by significantly low densities of immunogold labeling in the corresponding terminals of CAST-knockout (KO) mice (Fig. 1H, filled columns). This was also true for Nrxn, which was detected at comparable levels on the presynaptic membrane of the three types of terminals (Fig. 1 I and J and Fig. S1 K and L). Therefore, presynaptic molecules are recruited to the contact sites at dopaminergic terminals, like those at GABAergic and glutamatergic terminals. Taking these data together, this finding indicates that the presynaptic neurochemical phenotype at dopamine synapses is exclusively dopaminergic, and their contact sites exhibit presynaptic differentiation.
Dopamine Receptor Expression.
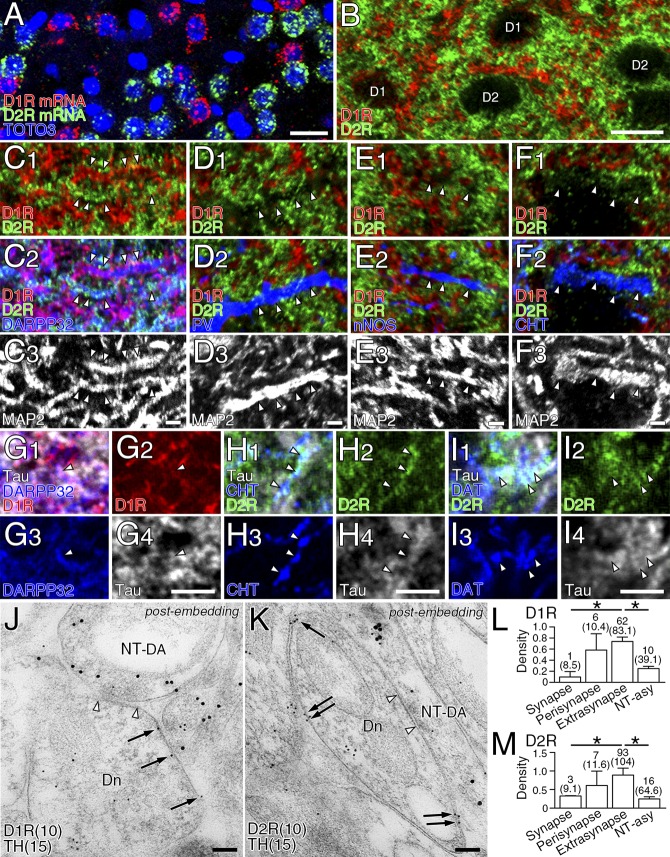
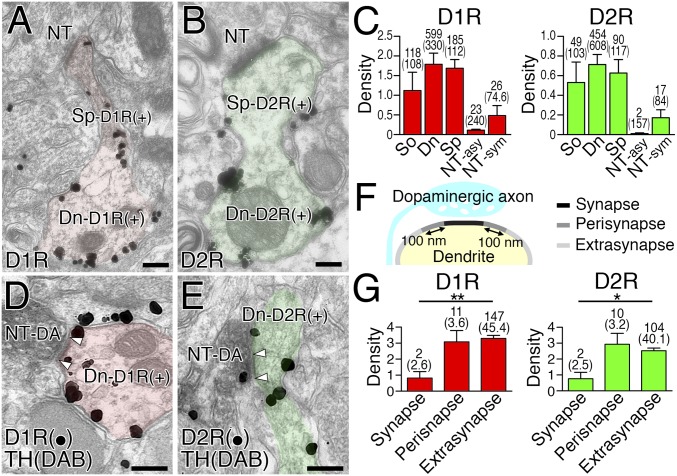
Before investigating postsynaptic neurochemical phenotypes, we examined the basic expression profile of dopamine receptors in the striatum. Expression of D1R and D2R was mutually exclusive at both the transcription and protein levels (Fig. S2 A and B), and detected predominantly along dendrites of MSNs labeled for 32-kDa dopamine- and cAMP-regulated neuronal phosphoprotein (DARPP32) (Fig. S2C). In comparison, D1R and D2R expression was low or undetectable in dendrites of GABAergic and cholinergic interneurons expressing their neuronal markers parvalbumin (PV), neuronal nitric oxide synthase (nNOS), and choline transporter (CHT) (Fig. S2 D–F). In MSNs, the density of cell membrane-attached metal particles for D1R and D2R was comparably high in the soma, dendritic shaft, and dendritic spines (Fig. 2 A–C). In the neuropil, weak immunolabeling for D1R and D2R was also observed in terminals forming symmetric—but not asymmetric—synapses (Fig. 2C). Triple immunofluorescence revealed low to moderate axonal immunolabeling for D1R in DARPP32+ MSN axons, and D2R in CHT-labeled cholinergic and DAT-labeled dopaminergic axons (Fig. S2 G–I).
Fig. S2.
Expression of D1R and D2R in striatal MSNs. (A) Fluorescent in situ hybridization for D1R (red) and D2R (green) mRNAs and nuclear counterstaining with TOTO-3 (blue). (B) Double immunofluorescence for D1R (red) and D2R (green). Cell bodies and neuropils are labeled for D1R (D1) and D2R (D2) in a segregated manner. (C–F) Quadruple immunofluorescence for D1R (red), D2R (green), MAP2 (white), and neuronal markers (blue), including DARPP32 (C), PV (D), nNOS (E), and CHT (F). In MAP2-labeled dendrites (arrowheads), DARPP32+ MSNs express either D1R or D2R at high levels (C), whereas various interneurons are low or negative in expression of dopamine receptors (D–F). (G) Triple immunofluorescence for D1R (red), tau (white), and DARPP32 (blue) showing weak expression of D1R in tau-labeled axons of DARPP32+ MSNs (arrowheads). (H and I) Triple immunofluorescence for D2R (green), tau (white), and CHT (H, blue) or DAT (I, blue) showing moderate expression of D2R in tau-labeled axons of CHT+ cholinergic (H, arrowheads) and DAT+ dopaminergic axons (I, arrowheads). (J and K) Double-label postembedding immunoelectron microscopy for TH [Ø (diameter) = 15-nm colloidal gold particles] and D1R (J, Ø = 10 nm) or D2R (K, Ø = 10 nm) showing predominant extrasynaptic and perisynaptic labeling (arrows) of dendrites (Dn) forming symmetric synapses (open arrowhead pairs) with TH+ dopaminergic terminals (NT-DA). Tissue specimens were mildly fixed with 0.2% picric acid/2% paraformaldehyde to increase the sensitivity of dopamine receptor detection. (L and M) Densities (mean ± SEM; n =3 mice for each) for D1R (L, 55 synapses) and D2R (M, 60 synapses) labelings per 1 µm of the synaptic, perisynaptic, and extrasynaptic membranes. The length of the plasma membrane (μm, in parentheses) and the number of metal particles analyzed are indicated above each column. *P < 0.05 (unpaired t test). [Scale bars: 20 μm (A and B), 2 μm (C–I), and 100 nm (J and K).]
Fig. 2.
Expression profiles of dopamine receptors in the striatum. (A and B) Pre-embedding immunoelectron microscopy for D1R (A) and D2R (B). D1R- and D2R-labeled spiny dendrites are colored red and green, respectively. (C) Labeling densities for D1R (Left) and D2R (Right) per 1 μm of the plasma membrane in somata (So), dendritic shafts (Dn), dendritic spines (Sp), and nerve terminals forming asymmetric (NT-asy) or symmetric (NT-sym) synapses. (D and E) Double-label pre-embedding immunoelectron microscopy for TH (DAB) and D1R (D, particles) or D2R (E, particles). Arrowhead pairs indicate dopamine synapses formed by TH-labeled dopaminergic terminals (NT-DA). (F) The synaptic (black), perisynaptic (dark gray), and extrasynaptic (light gray) membranes of dendrites around dopamine synapses. (G) Densities for D1R (Left, 18 synapses) and D2R (Right, 16 synapses) labelings per 1 µm of the synaptic, perisynaptic, and extrasynaptic membranes. The length of the plasma membrane (μm) and the number of metal particles analyzed are indicated in parentheses or above each column, respectively. Error bars represent SEM. *P < 0.05 and **P < 0.01 (unpaired t test). (Scale bars, 200 nm.)
Next, we examined the distribution of D1R and D2R in relation to dopamine synapses using double-label pre-embedding immunoelectron microscopy. Metal particles for D1R and D2R were densely distributed on the extrasynaptic surface of spiny dendrites, whereas the postsynaptic membrane in contact with TH-labeled dopaminergic terminals (diaminobenzidine precipitates) was rarely labeled (Fig. 2 D and E). We quantified this by measuring the density of metal particles on the synaptic, perisynaptic (<100 nm from the edge of the dopamine synapse), and extrasynaptic (>100 nm) membranes (Fig. 2F). The mean labeling density for D1R and D2R was significantly lower in the synaptic membrane than in the extrasynaptic membrane (Fig. 2G). To test the possibility of hindered antibody penetration into dopamine synapses, we performed postembedding immunoelectron microscopy using tissue specimens fixed mildly with 0.2% picric acid/2% paraformaldehyde fixative (Fig. S2 J and K). Again here, the synaptic membrane of TH-labeled dopamine synapses was significantly low for D1R or D2R labeling than the extrasynaptic membrane (Fig. S2 L and M). Thus, dopamine receptors are widely expressed on the extrasynaptic somatodendritic surface of MSNs, with no particular accumulation at—or gradient toward—dopamine synapses.
Postsynaptic Phenotypes at Dopamine Synapses.
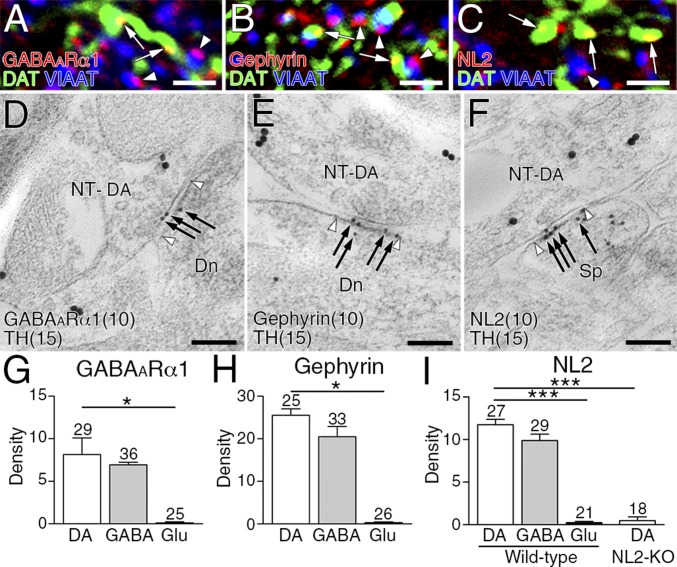
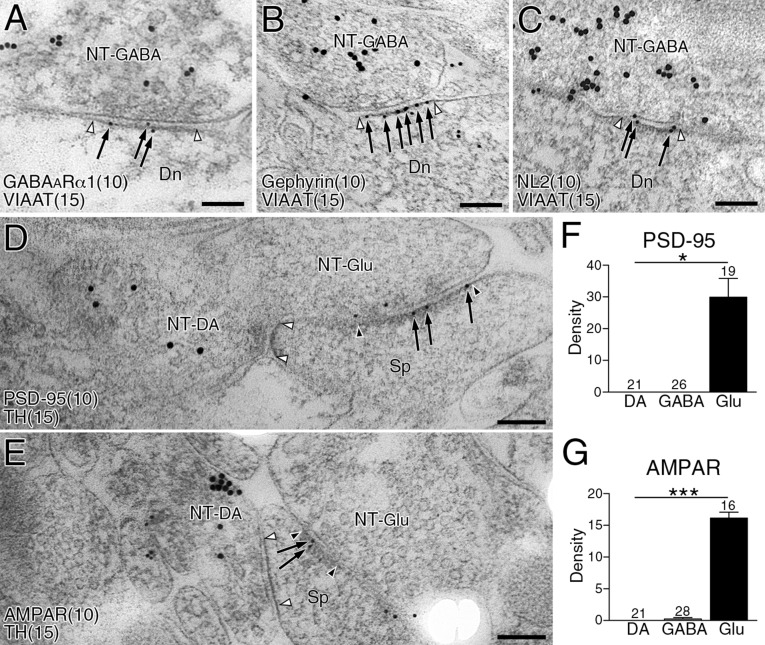
We then examined which types of molecules construct postsynaptic membrane specializations at dopamine synapses. From their symmetric nature, we examined expression levels of the following GABAergic postsynaptic proteins: GABAARα1, gephyrin (a scaffolding protein interacting with GABAA and glycine receptors) and NL2 (a synaptic adhesion protein interacting with GABAA receptors and gephyrin) (20, 21). All three proteins were clustered in the neuropil, and tightly apposed to VIAAT-labeled GABAergic (Fig. 3 A–C, arrowheads) and DAT-labeled dopaminergic (Fig. 3 A–C, arrows) terminals. Postsynaptic localization of these proteins was further tested by postembedding double-label immunoelectron microscopy (Fig. 3 D–F and Fig. S3 A–C). The density of immunogold labeling for GABAARα1, gephyrin, and NL2 was almost comparable between dopamine and GABAergic synapses, whereas the density at glutamatergic synapses was not different from background (Fig. 3 G–I). In contrast, glutamatergic postsynaptic proteins, PSD-95 and AMPA receptors, were enriched at glutamatergic synapses, but hardly detected at dopamine or GABAergic synapses (Fig. S3 D–G). Therefore, the postsynaptic phenotype at dopamine synapses is exclusively GABAergic.
Fig. 3.
GABAergic postsynaptic phenotype at striatal dopamine synapses. (A–C) Triple immunofluorescence for DAT (green) and VIAAT (blue), and for GABAARα1 (A, red), gephyrin (B, red), or NL2 (C, red). Note close apposition of GABAARα1, gephyrin, and NL2 clusters to both VIAAT+ GABAergic terminals (arrowheads) and DAT+ dopaminergic terminals (arrows). (D–F) Double-label postembedding immunoelectron microscopy for TH [Ø (diameter) = 15 nm] and for GABAARα1 (D, Ø = 10-nm colloidal gold particles), gephyrin (E, Ø = 10 nm), or NL2 (F, Ø = 10 nm). Immunogold particles (arrows) are concentrated at symmetric synapses formed by TH-labeled dopaminergic terminals (NT-DA, arrowhead pairs). (G–I) The density of immunogold labeling for GABAARα1 (G), gephyrin (H), or NL2 (I) per 1 μm of synaptic membrane at dopamine (DA), GABAergic (GABA), and glutamatergic (Glu) synapses. The specificity of NL2 labeling is confirmed by almost blank labeling in NL2-KO mice (I). Representative images of GABAergic synapses are shown in Fig. S3. Numbers of synapses analyzed are indicated above each column. Error bars represent SEM. *P < 0.05 and ***P < 0.001 (unpaired t test). [Scale bars: 2 μm (A–C) and 100 nm (D–F).]
Fig. S3.
Expression of GABAergic, but not glutamatergic, postsynaptic proteins at dopamine synapses. (A–C) Double-label postembedding immunoelectron microscopy for GABAARα1 [A, Ø (diameter) = 10-nm colloidal gold particles], gephyrin (B, Ø = 10 nm), or NL2 (C, Ø = 10 nm), and for VIAAT (Ø = 15 nm). Immunogold particles for GABAARα1, gephyrin, or NL2 (arrows) are concentrated at symmetric synapses formed by VIAAT-labeled GABAergic terminals (NT-GABA, open arrowhead pairs). (D and E) Double-label postembedding immunoelectron microscopy for TH (Ø = 15-nm colloidal gold particles) and PSD-95 (D, Ø = 10 nm), or AMPA receptor (AMPAR; E, Ø = 10 nm) in the striatum. Immunogold particles for PSD-95 and AMPAR (arrows) are found on the postsynaptic membrane at TH− asymmetric synapses on dendritic spines (glutamatergic, filled arrowhead pairs), but not at TH+ symmetric synapses (dopamine, open arrowhead pairs). Dn, dendrite; NT-DA, dopaminergic nerve terminal; NT-Glu, glutamatergic nerve terminal; Sp, spine. (F and G) The density (mean ± SEM; 3 mice for each) of immunogold labeling for PSD-95 (F) or AMPAR (G) per 1 µm of the synaptic membrane at dopamine (DA), GABAergic (GABA), and glutamatergic (Glu) synapses. The numbers of total synapses analyzed are indicated above each column. *P < 0.05, ***P < 0.001 (unpaired t test). (Scale bars, 100 nm.)
Postsynaptic Target of Dopamine Synapses.
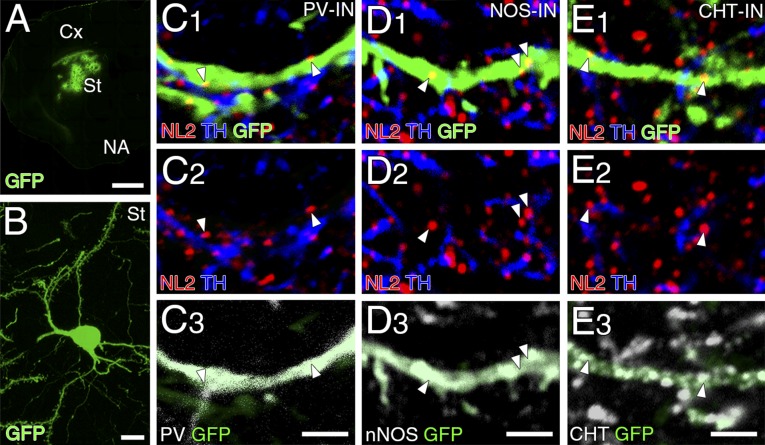
Dopamine synapses are frequently found in dendritic shafts and spines of MSNs (12, 13). In the present study, we quantitatively assessed target striatal neurons by lentivirus-mediated single neuronal labeling with GFP (Fig. S4 A and B). GFP-labeled dendrites (green) were further examined by immunofluorescence for TH (blue), NL2 (red), and neuronal markers (white) (Fig. 4 B and C and Fig. S4 C–E). The adenosine receptor A2AR was used as a marker for i-MSNs instead of D2R, because of its exclusive somatodendritic expression (22). The location of dopamine synapses was identified by the presence of NL2 clusters apposing TH+ dopaminergic terminals on GFP-labeled dendrites (Fig. 4A). These NL2-clustered dopamine synapses were richly expressed on spiny dendrites of both D1R+ d-MSNs and A2AR+ i-MSNs (Fig. 4 B and C, arrows), whereas they were rare on aspiny dendrites of GABAergic and cholinergic interneurons labeled for PV, nNOS, and CHT (Fig. S4 C–E). Instead, most NL2 clusters on these interneuron dendrites were judged to be non-dopamine synapses because they did not appose TH+ dopaminergic terminals (Fig. S4 C–E, arrowheads).
Fig. S4.
Dopamine synapses are sparse on dendrites of striatal interneurons. (A and B) Lentiviral GFP labeling of striatal neurons at low (A) and high magnifications (B, single striatal neuron). Cx, cortex; NA, nucleus accumbens; St, striatum. (C–E) Quadruple immunofluorescence for NL2 (red), GFP (green), TH (blue), and interneuron markers (white), including PV (C), nNOS (D), and CHT (E). Note that NL2 clusters on GFP-labeled interneuron dendrites (white arrowheads) rarely appose TH+ dopaminergic terminals. [Scale bars: 100 μm (A), 10 μm (B), and 2 µm (C–E).]
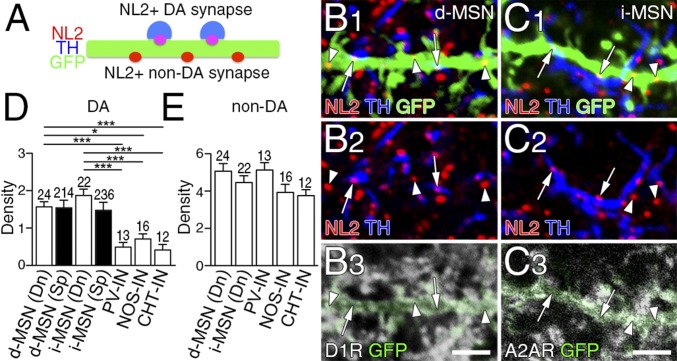
Fig. 4.
Dopamine synapses are preferentially formed on to dendrites of two types of MSNs. (A) Schematic to distinguish NL2-clustered dopamine and non-dopamine synapses on GFP-labeled dendrites of striatal neurons. (B and C) Quadruple immunofluorescence for NL2 (red), GFP (green), and TH (blue), and for D1R (B, white) or A2AR (C, white). NL2-clustered dopamine synapses (arrows) are preferentially distributed on dendrites of D1R-labeled d-MSNs (B) and A2AR-labeled i-MSNs (C). (D and E) The density of NL2-clustered dopamine (D) and non-dopamine (E) synapses per 10 μm of dendritic shafts (Dn) and spines (Sp) in d-MSNs and i-MSNs, and dendrites of striatal interneurons. Immunofluorescence images for striatal interneurons are shown in Fig. S4. The number of dendrites analyzed is indicated above each column. Error bars represent SEM. *P < 0.05 and ***P < 0.001 (one-way ANOVA with Tukey’s post hoc test). (Scale bars, 2 μm.)
We measured the density of NL2-clustered dopamine and non-dopamine synapses on MSNs. The density of dopamine synapses on dendritic shafts (0.75- to 1.5-µm diameter) in d-MSNs and i-MSNs was significantly (three- to fourfold) higher than that in interneurons (Fig. 4D, open columns), whereas that of nondopamine synapses showed no significant differences among striatal neurons (Fig. 4E). The density of dopamine synapses on dendritic spines was also comparable between the two types of MSNs (Fig. 4D, filled columns). Therefore, dendrites of d-MSNs and i-MSNs are targeted equally as a substrate for dopamine synapse formation.
NL2-Mediated Presynaptic Differentiation in Vitro.
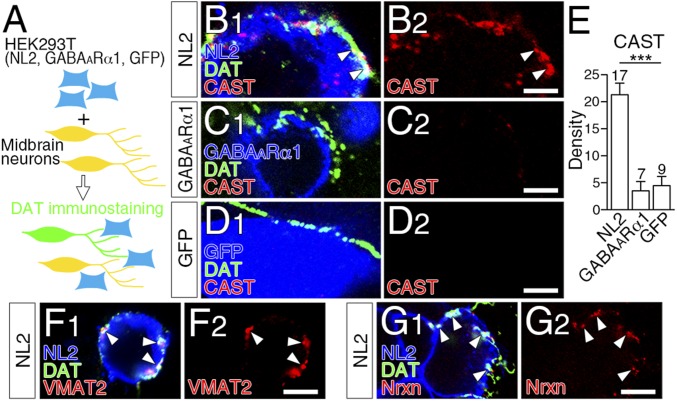
We hypothesized that GABAergic postsynaptic molecules mediated dopamine synapse formation, as they do for GABAergic synapse formation (21). To pursue this possibility, we cocultured primary midbrain or striatal neurons at 9–11 d in vitro with HEK293T cells expressing GABAARα1, NL2, or GFP, and examined whether presynaptic molecules were recruited to their contact sites (Fig. 5A and Fig. S5A). In this coculture assay, CAST, VMAT2, and Nrxn were robustly and significantly recruited to contact sites between DAT-labeled dopaminergic axons and HEK293T cells expressing NL2 (Fig. 5 B and E–G), but not GABAARα1 or GFP (Fig. 5 C and D). Similar presynaptic differentiation was observed in cocultures of striatal GABAergic neurons with HEK293T cells expressing NL2 (Fig. S5 B–E). Therefore, NL2 induces presynaptic differentiation in axons of midbrain dopamine neurons and striatal GABAergic neurons in vitro.
Fig. 5.
NL2-mediated presynaptic differentiation of dopaminergic axons in vitro. (A) Schematic of coculture assay of midbrain neurons with HEK293T cells expressing NL2, GABAARα1, or GFP. Axons of midbrain dopamine neurons were identified by DAT immunofluorescence. (B–D) Triple immunofluorescence for CAST (red) and DAT (green), and for NL2 (B, blue), GABAARα1 (C, blue) or GFP (D, blue). CAST clusters are recruited to contact sites of dopaminergic axons with HEK293T cells expressing NL2 (B, arrowheads), but not GABAARα1 (C) or GFP (D). (E) The density of CAST clusters per 100 μm of dopaminergic axon in contact with HEK293T cells. The number of HEK293T cells contacted by DAT-labeled dopaminergic axons is indicated above each column. Error bars represent SEM. ***P < 0.001 (Mann–Whitney u test). (F and G) Triple immunofluorescence for VMAT2 (F, red) or Nrxn (G, red), DAT (green), and NL2 (blue) in cocultures of midbrain dopamine neurons and HEK293T cells expressing NL2. (Scale bars, 2 μm.)
Fig. S5.
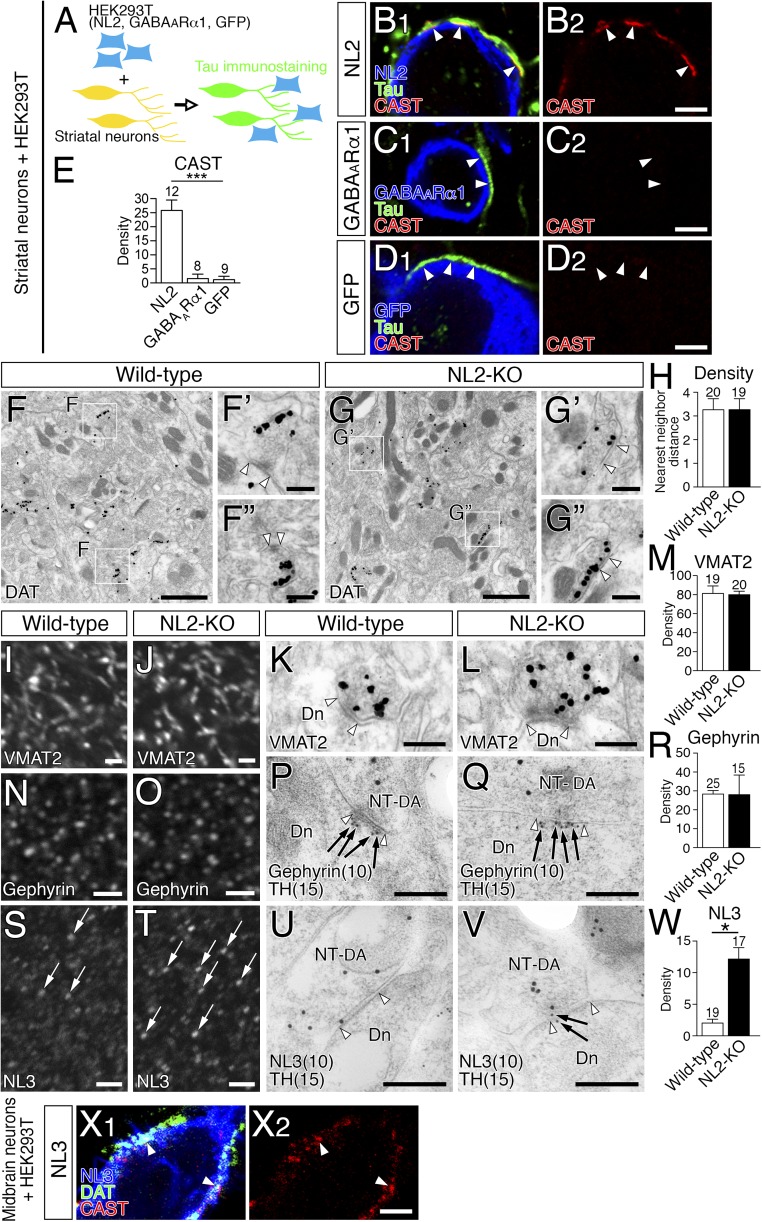
NL2-mediated presynaptic differentiation of GABAergic axons in vitro (A–E), unaffected dopamine synapse formation and compensatory NL3 up-regulation in the striatum of NL2-KO mice (F–W), and NL3-mediated presynaptic differentiation of dopaminergic axons in vitro (X). (A) A schematic illustration of coculture assay of striatal neurons with HEK293T cells transiently expressing NL2, GABAARα1, or GFP. Because striatal neurons are exclusively GABAergic, tau+ axons were discerned as GABAergic axons in this experiment. (B–D) Triple immunofluorescence for CAST (red) and DAT (green), and for NL2 (B, blue), GABAARα1 (C, blue) or GFP (D, blue). CAST clusters are recruited to contact sites (arrowheads) of striatal GABAergic axons with HEK293T cells expressing NL2 (B), but not GABAARα1 (C) or GFP (D). (E) The density of CAST clusters per 100 µm of striatal GABAergic axons in contact with HEK293T cells. The number of HEK293T cells contacted by tau-labeled striatal GABAergic axons analyzed is indicated above each column. ***P < 0.001 (one-way ANOVA with Tukey's post hoc test). (F and G) Pre-embedding immunoelectron microscopy for DAT in wild-type (F) and NL2-KO (G) mice. DAT+ dopamine synapses in the boxed areas of F or G are enlarged in F′ and F′′ or G′ and G′′, respectively. Open arrowhead pairs indicate symmetric contacts at DAT+ dopamine synapses. (H) Nearest-neighbor distances (µm) between dopamine synapses in wild-type (open column) and NL2-KO (filled column) mice. (I–W) Immunofluorescence and immunogold labeling for VMAT2 (I–M), gephyrin (N–R), and NL3 (S–W) in the striatum of wild-type (I, K, N, P, S, and U) and NL2-KO (J, L, O, Q, T, and V) mice. Pre-embedding immunoelectron microscopy was applied for VMAT2 (K and L), whereas double-label postembedding immunoelectron microscopy was used for TH [Ø (diameter) = 15-nm colloidal gold particles; P, Q, U, and V] and gephyrin (Ø = 10 nm; P and Q) or NL3 (Ø = 10 nm; U and V). Bar graphs show the densities (mean ± SEM; n = 3 mice for each) of VMAT2 labeling (M) per 1 µm2 of dopaminergic terminals and of gephyrin (R) or NL3 (W) labeling per 1 µm of dopamine synapses in wild-type (open columns) and NL2-KO (filled columns) mice. Note that immunofluorescence intensity of NL3 in wild-type mice is low to moderate (S), whereas that in NL2-KO mice is elevated in some clusters (T, arrows). Open arrowhead pairs indicate symmetric contacts at dopamine synapses. Dn, dendrite; NT-DA, dopaminergic nerve terminal. The numbers of total synapses analyzed are indicated above each column (H, M, R, and W). *P < 0.05 (unpaired t test). Error bars represent SEM. (X) Triple immunofluorescence for CAST (red), DAT (green), and NL3 (blue) in cocultures of midbrain dopamine neurons with HEK293T cells expressing NL3. Note NL3 expressed in HEK293T cells induces CAST clustering at contact sites with DAT-labeled dopaminergic axons (arrowheads). [Scale bars: 2 µm (B–D, I, J, N, O, S, T, and X), 1 µm (F and G), 200 nm (F′, F′′, G′, and G′′), and 100 nm (K, L, P, Q, U, and V).]
NL3 Up-Regulates at Dopamine Synapses in NL2-KO Mice.
To explore this role in vivo, changes in the density of dopamine synapses and molecular expression were investigated in NL2-KO mice. We assessed the density of DAT-labeled dopamine synapses by measuring their nearest-neighbor distances. No significant differences in dopamine synapse density were found between wild-type and NL2-KO mice (Fig. S5 F–H). Immunofluorescence and immunogold labelings for presynaptic VMAT2 (Fig. S5 I–M) and postsynaptic gephyrin (Fig. S5 N–R) were comparable at TH-labeled dopamine synapses between wild-type and NL2-KO mice, whereas significant up-regulation of NL3 occurred at dopamine synapses in NL2-KO mice (Fig. S5 S–W). In cocultures with HEK293T cells expressing NL3, NL3 also induced presynaptic differentiation in dopaminergic axons in vitro (Fig. S5X). Therefore, we concluded that the role of NL2 in dopamine synapse formation in vivo could not be addressed using NL2-KO mice, because of compensatory up-regulation of NL3.
Reciprocal Changes of Dopamine and GABAergic Synapses by NL2 Knockdown.
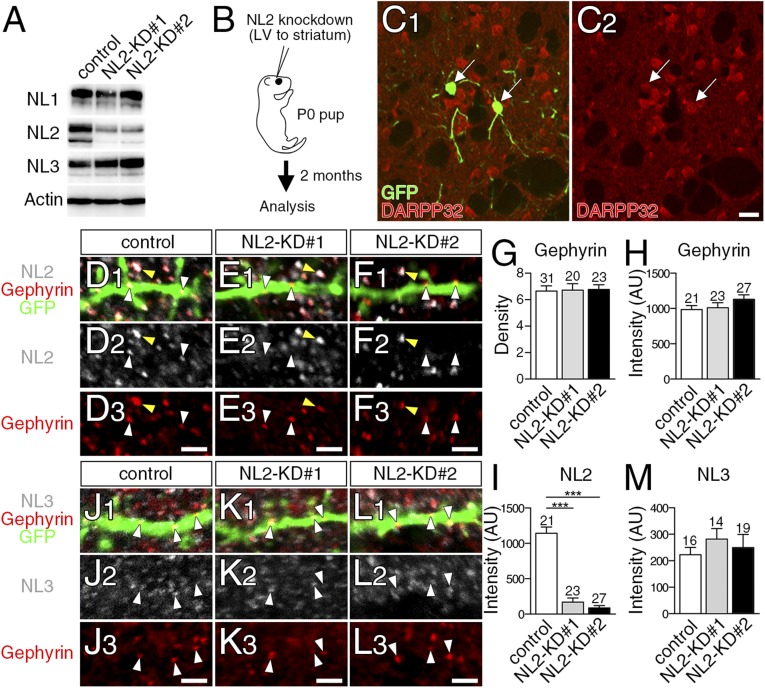
To overcome this problem, we used a sparse knockdown (KD) of NL2, which has been used to uncover the role of NL1 in cortical synaptogenesis (23). We prepared control-microRNA (miR), NL2-miR#1, and NL2-miR#2. Both NL2-miRs effectively and selectively reduced NL2 expression in HEK293T cells (Fig. S6A). We injected lentivirus vectors carrying GFP and one of the three miRs into the striatum of newborn pups (Fig. S6B). Two months after injection, a few MSNs expressed GFP (Fig. S6C) (12 of 765 DARPP32+ MSNs). We first checked NL2 expression at gephyrin clusters on GFP-labeled spiny dendrites. No significant changes were observed in the density or fluorescent intensity of gephyrin clusters between control-miR-infected (control) and NL2-miR–infected (NL2-KD) neurons (Fig. S6 D–F, red, and G and H). At gephyrin clusters, fluorescent intensity for NL2 was markedly and significantly lower in NL2-KD neurons (white arrowheads in Fig. S6 D–F and I), but not in neighboring noninfected neurons (Fig. S6 D–F, yellow arrowheads). Notably, the fluorescence intensity for NL3 at gephyrin clusters showed no significant differences between control and NL2-KD neurons (Fig. S6 J–L, white arrowheads, and M). Therefore, injection of NL2-miRs effectively reduces NL2 expression in MSNs without affecting the total number and intensity of gephyrin clusters or NL3 expression at gephyrin clusters.
Fig. S6.
Lentivirus-mediated sparse knockdown of NL2 in the striatum in vivo. (A) Immunoblot for NL2- KD efficiency and specificity in HEK293T cells expressing NL1, NL2, and NL3. (B) Lentiviral vectors (LV) were injected into the striatum at birth, and analysis was conducted at 2 mo of age. (C) Double immunofluorescence for GFP and DARPP32 in the adult striatum. Most of GFP-labeled neurons are DARPP32+ MSNs (arrows). (D–F and J–L) Triple immunofluorescence for NL2 (D–F, gray) or NL3 (J–L, gray), gephyrin (red), and GFP (green) in spiny dendrites of control (D and J), NL2-KD#1 (E and K), or NL2-KD#2 (F and L) neurons. White and yellow arrowheads indicate gephyrin clusters on infected (GFP-labeled) and noninfected (GFP-unlabeled) dendrites, respectively. (G–I and M) The density of gephyrin clusters per 10 µm of control and KD dendrites (G), and fluorescence intensity (arbitrary units) of gephyrin (H), NL2 (I), and NL3 (M) clusters. Total numbers of dendrites (G) or gephyrin clusters (H, I, and M) analyzed are indicated above each column. Error bars represent SEM. ***P < 0.001 (Mann–Whitney u test). [Scale bars: 20 μm (C) and 2 µm (D–F and J–L).]
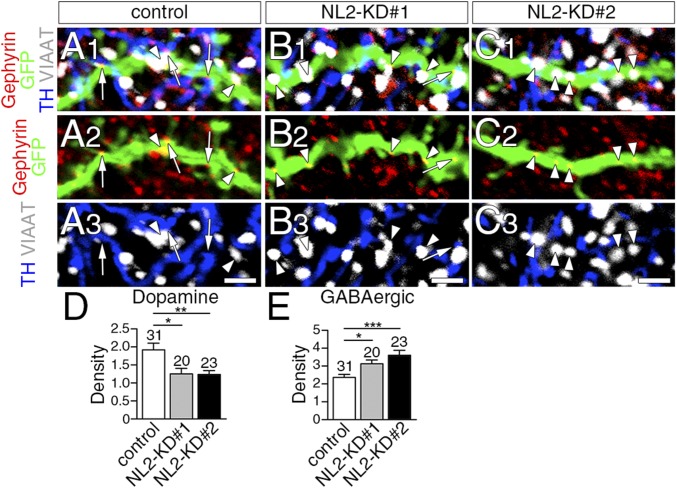
Next, we analyzed the density of dopamine and GABAergic synapses by counting the number of gephyrin clusters on GFP-labeled spiny dendrites that apposed TH+ dopaminergic terminals (Fig. 6 A–C, arrows) or VIAAT+ GABAergic terminals (Fig. 6 A–C, arrowheads), respectively. In NL2-KD neurons, the density of dopamine synapses was significantly lower than in control neurons (Fig. 6D), whereas that of GABAergic synapses was significantly greater (Fig. 6E).
Fig. 6.
Decrease of dopamine synapses and reciprocal increase of GABAergic synapses after sparse NL2 knockdown in striatal MSNs. (A–C) Quadruple immunofluorescence for gephyrin (red), GFP (green), TH (blue), and VIAAT (gray) in spiny dendrites of control (A), NL2-KD#1 (B), or NL2-KD#2 (C) neurons. Arrows and arrowheads indicate dopamine and GABAergic synapses, respectively, on GFP-labeled dendrites. (D and E) The density of dopamine (D) and GABAergic (E) synapses per 10 µm of control and KD dendrites. The total number of dendrites analyzed is indicated above each column. Error bars represent SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 (Mann–Whitney u test). (Scale bars, 2 μm.)
Discussion
In the present study, we have shown that striatal dopamine synapses are neurochemically mismatched contacts, and NL2 is involved in their formation.
Neurochemically Mismatched Contact at Dopamine Synapses.
Dopaminergic terminals were extensively colabeled for TH, DAT, and VMAT2, which are essential for the synthesis, recycling, and vesicular filling of dopamine (24). Moreover, CAST and Nrxn, which are involved in active zone formation and synaptic adhesion, respectively (6, 25), accumulated on the presynaptic membrane at dopamine synapses and were expressed at levels comparable to conventional glutamatergic and GABAergic synapses. This orchestrated molecular architecture consolidates the dopaminergic phenotype in the presynaptic side of dopamine synapses. Surprisingly, GABAergic proteins GABAARα1, gephyrin, and NL2 were expressed in the postsynaptic side at densities comparable to those at conventional GABAergic synapses. Therefore, dopamine synapses are contacts between dopaminergic presynaptic and GABAergic postsynaptic structures. It has been reported that 40.7% of symmetric synapses expressing GABAA receptors in the striatum are formed by terminals in which GABA is low or undetectable (26). We assume that they represent most, if not all, dopamine synapses.
An increasing number of reports is emerging of cases in which two or more classic transmitters are coreleased at single synapses (27), and dopamine has been shown to be coreleased with glutamate or GABA (27, 28). However, we found no significant immunoreactivity for GABA, GAD, or VIAAT at dopaminergic terminals. The scarcity of GABA in dopaminergic terminals is consistent with previous studies reporting that no or few (11–13%) dopaminergic terminals are significantly labeled for GABA (18, 29). This finding suggests that GABA is coreleased little, if at all, at most dopamine synapses. Nevertheless, optogenetic stimulation of dopaminergic axons evokes GABAA receptor-mediated postsynaptic currents in MSNs through GAT1-mediated uptake and VMAT2-dependent vesicular filling of GABA (28, 30). In the present study, we observed weak expression of GAT1, as well as intense expression of VMAT2 in dopaminergic terminals. Taken together, this evidence indicates that the phenotypes of the major transmitters and receptors are neurochemically mismatched at dopamine synapses, but transporter-mediated GABA release could be conducted from dopaminergic terminals whose GABA content is undetectable by conventional immunohistochemistry or from a small subpopulation of dopaminergic terminals expressing high content of GABA.
NL-Mediated Dopamine Synapse Formation.
Synapse-type–specific transsynaptic interaction between NLs and Nrxns plays a key role in bidirectional synaptic differentiation (6). NL2 is selectively expressed at inhibitory synapses (8), binds gephyrin, and promotes its membrane targeting through collybistin activation (31). NL2 also interacts with GABAA receptors (20). These interactions underlie NL2-mediated specification of inhibitory synapses (9). Expression of NL2 at dopamine synapses, together with that of GABAARα1 and gephyrin, is thus consistent with the framework of synapse-type–dependent expression of the NL family. Our finding that significant amounts of CAST, VMAT2, and Nrxn were recruited to contact sites between dopaminergic axons and NL2-expressing HEK293T cells indicates that NL2 functions as a presynaptic organizer for dopaminergic axons in vitro, similarly to GABAergic axons (20, 32).
In vitro experiments clearly demonstrate that manipulations that up- or down-regulate NL expression alter synapse formation positively or negatively, respectively, indicating that NLs are intrinsically synaptogenic (9, 33). However, there is little or no change in the number and structure of synapses between global NL-KO and wild-type mice (23, 34). Indeed, we found no significant changes in the density of dopamine synapses in the striatum of NL2-KO mice. These conflicting results between the in vitro and in vivo conditions seem to be ascribed to the redundancy in the molecular form of NLs and Nrxns and their interactions (6). In support of this notion, NL3 was up-regulated at dopamine synapses in NL2-KO mice, which would mask synaptogenic actions by NL2. To overcome this problem, we adopted the sparse NL2-KD strategy to minimize compensatory up-regulation of NL3. Under this condition, the density of dopamine and GABAergic synapses on MSN dendrites was significantly decreased and increased, respectively, without a change in their total density. This finding suggests that NL2 mediates striatal synapse formation by giving competitive advantage to heterologous dopamine synapses over conventional GABAergic synapses. Therefore, NL2 appears to regulate the formation of striatal synapses in a competitive and input-dependent manner in vivo rather than in a simple synaptogenic manner in vitro. Such NL-dependent competitive synaptogenesis has also been reported for NL1 in the cortex, where cortical synapse formation is sensitive to sparse NL1-KD, causing transcellular differences in the relative amount of NL1 (23). Thus, different relative amounts of NL2 caused by sparse KD may also affect competitive synaptogenic processes between NL2-KD and control MSNs.
Postulated Role of Dopamine Synapses.
D1R and D2R are widely expressed on the extrasynaptic surface of MSN dendrites (15–17). The present immunohistochemistry confirmed this, and further clarified the lack of dopamine receptor accumulation at dopamine synapses. The mismatching between dopamine release and reception sites indicates that dopamine synapses are neither the neural device for the wired transmission that is conducted for fast and point-to-point signaling typical to conventional glutamatergic and GABAergic synapses, nor that for the volume transmission for slow and global modulation, such as muscarinic M1-mediated cholinergic transmission in the hippocampus (35, 36). Then, what is the role of neurochemically mismatched dopamine synapses?
Glutamatergic transmission to MSNs by cortical and thalamic inputs, and its modulation by dopaminergic, cholinergic, and GABAergic inputs, are the basis of functional regulation in the basal ganglia (10, 14). The diffusion model of quantal dopamine release postulates an effective radius of ∼2 and 7 µm for the activation of D1R and D2R, respectively (37). A recent study using a D2R biosensor points out that the spatiotemporal extent of dopaminergic transmission is more limited and D2R on MSNs functionally behaves as low-affinity receptors for fast dopaminergic transmission (38). Considering that MSN dendrites are both the preferential postsynaptic target of dopamine synapse formation and the neuronal element enriched with dopamine receptors, we suggest that such heterologous contacts function as a device to increase the target specificity and potency of dopaminergic modulation by anchoring dopamine release sites to dopamine-sensing neurons. In this regard, it should be noted that synaptic contacts are also formed by other neuromodulatory neurons (39–41). Of these, NL2 is expressed at symmetric synapses formed by cholinergic neurons in the forebrain (42). The possibility that NL2-dependent formation of neurochemically mismatched contacts could be the general strategy to attract neuromodulatory inputs to specific targets is an intriguing issue to be explored in future studies.
Materials and Methods
All animal experiments were approved by the Hokkaido Univeristy Animal Care and Use Committee. In the present study, we performed immunoblot, immunohistochemistry, lentiviral experiments, and coculture assay. More information about the experimental procedures, the specificity and combination of antibodies, and the labeling density of synaptic molecules in individual mice is available in SI Materials and Methods, Fig. S7, and Tables S1–S3.
Fig. S7.
Specificity of primary antibodies used in the present study. (A–C) VMAT2, GAD, and VIAAT antibodies. Patterns of immunofluorescence labeling for VMAT2 (A), GAD (B), and VIAAT (C) are shown in coronal sections through the striatum. (D–F) CAST antibody. Specific detection of CAST band at 130 kDa in immunoblot (D) and specific immunofluorescence-labeling of CAST (red) in synaptophysin-labeled nerve terminals (green) (E and F) are shown in brain tissues from wild-type mice, but not CAST-KO mice. (G and H) Nrxn1α antibody. Because of high sequence homology in the C terminus of Nrxn1α–3α (G), immunoblot detects 120- to 150-kDa bands in brain homogenates and HEK293T cell lysates transfected with Nrxn1α, -2α, and -3α (H). (I–L) D1R and D2R antibodies. Note the lack of immunofluorescence signals for D1R and D2R in brains of D1R-KO and D2R-KO mice, respectively. (M–Q) DARPP32 antibody. Immunoblot detects a 32-kDa band in both brain homogenates and HEK293T cell lysates expressing DARPP32 (M). Note similar patterns of labeling by fluorescent in situ hybridization for DARPP32 mRNA (N) and immunohistochemistry for DARPP32 (O) in parasagittal brain sections. Double immunofluorescence for DARPP32 (P and Q, red) and PV (P, green) or CHT (Q, green) shows the lack of DARPP32 labeling in PV- and CHT-expressing interneurons (asterisks). (R–T) NL1, NL2, and NL3 antibodies. Immunoblot with NL1 (Upper), NL2 (Middle), and NL3 (Bottom) antibodies selectively detects 100–150-kDa protein bands in brain homogenates and HEK293T cell lysates expressing NL1–4 (R). Double immunofluorescence shows that NL2 (green) overlaps well with gephyrin (red) in the striatum (S). Triple immunofluorescence for NL2 (green), NL3 (red), and DAT (blue) shows that NL3 is expressed at both NL2+/DAT- synapses (yellow arrows) and NL2+/DAT+ dopamine synapses (white arrows) (T). Cb, cerebellum; Cx, cortex; Hi, hippocampus; MO, medulla oblongata; NA, nucleus accumbens; St, striatum; Th, thalamus. [Scale bars: 1 mm (A–C, I–L, N, and O), 20 μm (P and Q), and 2 μm (E, F, S, and T).]
Table S1.
List of primary antibodies used in the present study
| Molecule | Sequence (NCBI #) | Host | Specificity | Source |
| A2AR | 388–410 (NM009630) | GP/Go | IB, KO | (22) |
| AMPAR | 727–745 (X57497) | GP | IB/HEK | (46) |
| CAST | 113–161 (NM_178085) | Rb/GP | IB, KO | Present study |
| CHT | 531–580 (BC065089) | Go/GP | IB | (36) |
| D1R | 402–446 (NM010076) | GP/Go | IB, PT | (22) |
| D2R | 271–370 (NM010077) | Rb | IB, PT | (22) |
| DARPP32 | 163–194 (NM_144828) | Rb/GP | IB/HEK | Present study |
| DAT | 1–60 (BC054119) | Rb/GP/Go | IB/HEK | (47) |
| GABA | Ms | Sigma (A0310) (48) | ||
| GABAARα1 | 369–386 (NM_010250) | Rb/GP | IB/HEK | (49) |
| GAD65/67 | 268–593 (A28072) | Go | IB, PT | (48) |
| GAT1 | 1–46/564–599 (NM_178703) | Rb/GP | IB/HEK | (49) |
| Gephyrin | 54–94 (NM_1729529) | Rb | IB/HEK | (49) |
| Ms | Synaptic Systems (#147111) | |||
| GFP | 1–238 (YP_002302326) | Rb/GP/Go | KI | Frontier Institute |
| MAP2 | Ms | Millipore (MAB3418) | ||
| NL1 | 46–69 (NM_138666.3) | Rb | IB/HEK | Present study |
| NL2 | 784–829 (NP_12455) | Rb/GP | IB/HEK | (49) |
| NL3 | 768–793 (NM_172932) | Rb/GP | IB/HEK | Present study |
| nNOS | 1400–1429 (NM_008712) | GP | IB | (47) |
| Nrxn1α | 1454–1507 (NM_020252) | Rb | IB/HEK, *1 | Present study, |
| PSD-95 | 1–64 (D50621) | Rb/GP | IB | (50) |
| PV | 1–110 (NM_013645) | Rb/GP/Go | (51) | |
| synaptophysin | Rb/GP | IB | (50) | |
| TH | Ms | Immunostar (#22941) | ||
| Rb | Millipore (AB152) | |||
| VGluT1 | 531–560 aa (BC054462) | Go | IB | (51) |
| VGluT2 | 559–582 aa (BC038375) | Go | IB | (51) |
| VGluT3 | 558–602 aa (AF510321) | Go | IB | (52) |
| VIAAT | 31–112 (BC052020) | Rb/GP/Go | (51) | |
| VMAT2 | 468–515 (L00603) | Rb | IB | (52) |
| Tau | Bovine tau | Ms | Millipore (MAB3420) |
A2AR, adenosine A2 receptor; AMPAR, AMPA receptor; CAST, cytomatrix protein at the active zone, also known as ERC2; CHT, high-affinity choline transporter; D1R/D2R, dopamine receptor-1/2; DARPP32, dopamine- and cAMP-regulated neuronal phosphoprotein or protein phosphatase 1, regulatory (inhibitory) subunit 1B; DAT, plasmalemmal dopamine transporter; GAD65/67, 65/67-kDa glutamic acid decarboxylase; GABA, γ-aminobutyric acid; GAT1, plasmalemmal GABA transporter-1; gephyrin, scaffold protein interacting with glycine and GABAA receptors; GFP, green fluorescent protein; Go, goat polyclonal antibody; GP, guinea pig polyclonal antibody; IB, immunoblot with brain homogenates; IB/HEK, immunoblot with transfected HEK293T cell lysates; KI, specific neuronal labeling in GAD67-GFP knock-in, but not wild-type, mice; KO, no signal in knockout mouse brain; MAP2, microtubule-associated protein-2; Ms, mouse monoclonal antibody; NL1–3, neuroligin-1–3; Nrxn1α, neurexin-1α; nNOS, neuronal nitric oxide synthase; PSD-95, postsynaptic density protein-95; PT, preabsorption test; PV, parvalbumin; TH, tyrosine hydroxylase; VGluT1–3, type 1–3 vesicular glutamate transporter; VIAAT, vesicular inhibitory amino acid transporter; VMAT2, vesicular monoamine transporter-2.
1, Nrxn1α antibody also recognizes Nrxn2α and Nrxn3α owing to high C-terminus homology (Fig. S7H).
Table S3.
The labeling density of synaptic molecules in individual mice
| Mouse | Synapse type | Molecule | Synapse # | Labeling density (mean ± SEM) | Figure |
| WT #1 | DA | GABA | 9 | 61.7 ± 11.6 | 1F |
| WT #2 | DA | GABA | 5 | 38.2 ± 8.20 | 1F |
| WT #3 | DA | GABA | 6 | 19.4 ± 3.24 | 1F |
| WT #1 | GABA | GABA | 9 | 594 ± 109 | 1F |
| WT #2 | GABA | GABA | 5 | 336 ± 68.8 | 1F |
| WT #3 | GABA | GABA | 6 | 284 ± 40.8 | 1F |
| WT #1 | Glu | GABA | 11 | 40.7 ± 22.9 | 1F |
| WT #2 | Glu | GABA | 9 | 16.5 ± 7.01 | 1F |
| WT #3 | Glu | GABA | 5 | 8.03 ± 3.51 | 1F |
| WT #4 | DA | CAST | 15 | 4.26 ± 2.19 | 1H |
| WT #5 | DA | CAST | 15 | 4.38 ± 0.776 | 1H |
| WT #6 | DA | CAST | 13 | 1.76 ± 0.776 | 1H |
| CAST-KO #1 | DA | CAST | 10 | 0 ± 0 | 1H |
| CAST-KO #2 | DA | CAST | 11 | 0.57 ± 0.57 | 1H |
| CAST-KO #3 | DA | CAST | 14 | 0 ± 0 | 1H |
| WT #4 | GABA | CAST | 9 | 1.28 ± 0.86 | 1H |
| WT #5 | GABA | CAST | 9 | 1.15 ± 0.82 | 1H |
| WT #6 | GABA | CAST | 10 | 2.45 ± 1.39 | 1H |
| CAST-KO #1 | GABA | CAST | 12 | 0 ± 0 | 1H |
| CAST-KO #2 | GABA | CAST | 12 | 0.49 ± 0.49 | 1H |
| CAST-KO #3 | GABA | CAST | 8 | 0 ± 0 | 1H |
| WT #4 | Glu | CAST | 11 | 40.7 ± 22.9 | 1H |
| WT #5 | Glu | CAST | 8 | 16.5 ± 7.01 | 1H |
| WT #6 | Glu | CAST | 5 | 8.03 ± 3.51 | 1H |
| CAST-KO #1 | Glu | CAST | 5 | 0 ± 0 | 1H |
| CAST-KO #2 | Glu | CAST | 9 | 0 ± 0 | 1H |
| CAST-KO #3 | Glu | CAST | 8 | 0 ± 0 | 1H |
| WT #7 | DA | Nrxn | 5 | 7.75 ± 3.87 | 1J |
| WT #8 | DA | Nrxn | 7 | 1.94 ± 1.26 | 1J |
| WT #9 | DA | Nrxn | 10 | 3.54 ± 1.84 | 1J |
| WT #7 | GABA | Nrxn | 8 | 5.34 ± 3.91 | 1J |
| WT #8 | GABA | Nrxn | 7 | 2.92 ± 1.39 | 1J |
| WT #9 | GABA | Nrxn | 10 | 1.92 ± 0.866 | 1J |
| WT #7 | Glu | Nrxn | 6 | 4.91 ± 1.63 | 1J |
| WT #8 | Glu | Nrxn | 8 | 2.90 ± 1.15 | 1J |
| WT #9 | Glu | Nrxn | 7 | 3.84 ± 1.91 | 1J |
| WT #7 | DA | GABAARα1 | 12 | 8.87 ± 4.00 | 3G |
| WT #8 | DA | GABAARα1 | 10 | 11.1 ± 4.19 | 3G |
| WT #9 | DA | GABAARα1 | 10 | 4.47 ± 2.53 | 3G |
| WT #7 | GABA | GABAARα1 | 11 | 6.59 ± 1.97 | 3G |
| WT #8 | GABA | GABAARα1 | 13 | 7.54 ± 1.81 | 3G |
| WT #9 | GABA | GABAARα1 | 12 | 6.69 ± 1.83 | 3G |
| WT #7 | Glu | GABAARα1 | 7 | 0 ± 0 | 3G |
| WT #8 | Glu | GABAARα1 | 7 | 0 ± 0 | 3G |
| WT #9 | Glu | GABAARα1 | 11 | 0.197 ± 0.197 | 3G |
| WT #7 | DA | Gephyrin | 10 | 31.7 ± 8.19 | 3H, S5R |
| WT #8 | DA | Gephyrin | 7 | 25.5 ± 8.22 | 3H, S5R |
| WT #9 | DA | Gephyrin | 8 | 27.9 ± 4.20 | 3H, S5R |
| NL2-KO #1 | DA | Gephyrin | 5 | 28.3 ± 11.1 | S5R |
| NL2-KO #2 | DA | Gephyrin | 5 | 45.8 ± 16.0 | S5R |
| NL2-KO #3 | DA | Gephyrin | 5 | 10.0 ± 2.98 | S5R |
| WT #7 | GABA | Gephyrin | 11 | 29.7 ± 6.02 | 3H |
| WT #8 | GABA | Gephyrin | 12 | 15.9 ± 4.36 | 3H |
| WT #9 | GABA | Gephyrin | 10 | 22.0 ± 5.95 | 3H |
| WT #7 | Glu | Gephyrin | 5 | 0 ± 0 | 3H |
| WT #8 | Glu | Gephyrin | 6 | 0.48 ± 0.48 | 3H |
| WT #9 | Glu | Gephyrin | 10 | 0 ± 0 | 3H |
| WT #7 | DA | NL2 | 11 | 12.0 ± 4.60 | 3I |
| WT #8 | DA | NL2 | 6 | 12.0 ± 4.30 | 3I |
| WT #9 | DA | NL2 | 9 | 13.5 ± 4.07 | 3I |
| NL2-KO #1 | DA | NL2 | 5 | 0 ± 0 | 3I |
| NL2-KO #2 | DA | NL2 | 7 | 0 ± 0 | 3I |
| NL2-KO #3 | DA | NL2 | 6 | 0.991 ± 0.991 | 3I |
| WT #7 | GABA | NL2 | 7 | 10.8 ± 4.49 | 3I |
| WT #8 | GABA | NL2 | 12 | 8.39 ± 1.99 | 3I |
| WT #9 | GABA | NL2 | 10 | 10.5 ± 2.32 | 3I |
| WT #7 | Glu | NL2 | 8 | 0.68 ± 0.54 | 3I |
| WT #8 | Glu | NL2 | 6 | 0 ± 0 | 3I |
| WT #9 | Glu | NL2 | 7 | 0.326 ± 0.326 | 3I |
| WT #7 | DA | PSD-95 | 8 | 0 ± 0 | S3F |
| WT #8 | DA | PSD-95 | 6 | 0 ± 0 | S3F |
| WT #9 | DA | PSD-95 | 7 | 0 ± 0 | S3F |
| WT #7 | GABA | PSD-95 | 5 | 0 ± 0 | S3F |
| WT #8 | GABA | PSD-95 | 9 | 0 ± 0 | S3F |
| WT #9 | GABA | PSD-95 | 10 | 0 ± 0 | S3F |
| WT #7 | Glu | PSD-95 | 5 | 25.6 ± 6.15 | S3F |
| WT #8 | Glu | PSD-95 | 5 | 19.0 ± 7.12 | S3F |
| WT #9 | Glu | PSD-95 | 9 | 40.8 ± 5.65 | S3F |
| WT #7 | DA | AMPAR | 6 | 0 ± 0 | S3G |
| WT #8 | DA | AMPAR | 9 | 0 ± 0 | S3G |
| WT #9 | DA | AMPAR | 6 | 0 ± 0 | S3G |
| WT #7 | GABA | AMPAR | 6 | 0 ± 0 | S3G |
| WT #8 | GABA | AMPAR | 11 | 0.939 ± 0.939 | S3G |
| WT #9 | GABA | AMPAR | 11 | 0 ± 0 | S3G |
| WT #7 | Glu | AMPAR | 6 | 13.7 ± 3.34 | S3G |
| WT #8 | Glu | AMPAR | 5 | 16.4 ± 5.95 | S3G |
| WT #9 | Glu | AMPAR | 5 | 18.7 ± 7.79 | S3G |
| WT #10 | DA | VMAT2 | 7 | 78.9 ± 8.30 | S5M |
| WT #11 | DA | VMAT2 | 6 | 86.9 ± 13.2 | S5M |
| WT #12 | DA | VMAT2 | 6 | 74.2 ± 16.5 | S5M |
| NL2-KO #4 | DA | VMAT2 | 7 | 83.7 ± 15.3 | S5M |
| NL2-KO #5 | DA | VMAT2 | 7 | 66.8 ± 5.75 | S5M |
| NL2-KO #6 | DA | VMAT2 | 6 | 93.5 ± 16.2 | S5M |
| WT #7 | DA | NL3 | 8 | 1.06 ± 1.06 | S5W |
| WT #8 | DA | NL3 | 6 | 3.17 ± 2.02 | S5W |
| WT #9 | DA | NL3 | 5 | 1.85 ± 1.85 | S5W |
| NL2-KO #1 | DA | NL3 | 6 | 9.03 ± 4.37 | S5W |
| NL2-KO #2 | DA | NL3 | 6 | 12.2 ± 10.7 | S5W |
| NL2-KO #3 | DA | NL3 | 5 | 15.2 ± 4.16 | S5W |
DA, dopamine synapse; GABA, GABAergic synapse, Glu, glutamatergic synapses; WT, wild-type mouse. The density for GABA and VMAT2 is represented as the number of metal particles per 1 µm2 of the terminal. The density for other molecules is represented as the number of metal particles per 1 µm of the presynaptic or postsynaptic membrane.
SI Materials and Methods
Animals.
All animal experiments were approved by the Hokkaido University Animal Care and Use Committee. The dorsolateral portion of the striatum in adult male C57BL/6N, NL2-KO (Jackson Laboratory), CAST-KO (43), D1R-KO (44), and D2R-KO (45) mice (1–3 mo of age) was analyzed. D1R-KO and D2R-KO mice were homozygous for a knock-in mutation in which the human interleukin-2 receptor α-subunit gene was inserted into the D1R and D2R genes, respectively. Wild-type and KO littermates were analyzed for comparison. Both the qualitative and quantitative data were obtained from three mice in each animal group.
Fixation.
Under deep pentobarbital anesthesia (100 mg/kg of body weight, i.p.), mice were perfused transcardially with 60–75 mL of 4% (wt/vol) paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.2) for 10 min, unless otherwise noted. Striatal tissues perfused with 4% paraformaldehyde/0.1% glutaraldehyde in PB or 2% (wt/vol) paraformaldehyde/0.2% picric acid in PB were also used to detect GABA or dopamine receptors, respectively, by postembedding immunoelectron microscopy.
Section Preparation.
For conventional immunofluorescence and pre-embedding immunoelectron microscopy, sections (20 or 50 μm in thickness) were prepared on a microslicer (VT1000S; Leica) and processed for free-floating incubations. Ultrathin sections (100 nm) were prepared using an ultracryomicrotome (EM-FCS; Leica) after cryoprotection with 0.32M sucrose in PB, and mounted on silane-coated glass slides. For postembedding immunogold electron microscopy, microslicer sections (300 µm) were cryoprotected with 30% (vol/vol) glycerol in PB and frozen rapidly with liquid propane in the EM CPC unit (Leica). Frozen sections were immersed in 0.5% uranyl acetate in methanol at −90 °C in the AFS freeze-substitution unit (Leica), infiltrated at −45 °C with Lowicryl HM20 resin (Electron Microscopy Sciences), and polymerized with UV irradiation. Ultrathin sections were cut with an ultramicrotome (Ultracut; Leica) and mounted on formvar-coated nickel mesh grids. For fluorescent in situ hybridization, brains were removed from the skull under deep pentobarbital anesthesia and frozen immediately in powdered dry ice. Fresh-frozen sections (20 μm) were cut with a cryostat (CM1900; Leica).
Fluorescent in Situ Hybridization.
We used the following fluorescein- or digoxigenin-labeled riboprobes: mouse D1R (1-950, NM_010076), mouse D2R (416-1105, NM_010077), and mouse dopamine- and cAMP-regulated neuronal phosphoprotein or protein phosphatase DARPP32 (298-1000; NM_144828). Riboprobe synthesis and in situ hybridization were performed following the protocol described previously (36). Nuclear counterstaining was performed using TOTO-3 (Life Technologies). Images were captured using a confocal laser-scanning microscope equipped with a HeNe/Ar laser, and UPLSAPO 10×/0.40 and 20×/0.75 objective lenses (FV1000; Olympus). The specificity of hybridization signals was confirmed by a lack of any significant labeling using the sense riboprobes.
Antibody.
Information on the antigens, host species, sources, specificity, and source or references of primary antibodies is summarized in Fig. S7 and Table S1. Briefly, overall patterns of immunofluorescence labeling in coronal striatal sections are provided for TH (Fig. 1A), plasmalemmal DAT (Fig. 1B), VMAT2 (Fig. S7A), GAD65/67 (Fig. S7B), and VIAAT (Fig. S7C). The specificity of main primary antibodies is shown for cytomatrix protein at the active zone CAST by blank labelings by immunoblot and immunohistochemistry in CAST-KO mice (Fig. 1H and Fig. S7 D–F); Nrxns by detection of >150-kDa protein bands in the brain and HEK293T cell lysates transfected with Nrxn1α–3α (Fig. S7 G and H); D1R and D2R by the lack of immunolabeling in D1R-KO and D2R-KO mice (Fig. S7 I–L); DARPP32 by selective detection of a 32-kDa protein band in the brain and HEK293T cell lysates transfected with DARPP32, similar patterns of DARPP32 immunohistochemistry and in situ hybridization, and lack of DARPP32 immunolabeling in striatal interneurons (Fig. S7 M–Q); gepherin by extensive overlap with NL2 clusters in the striatum and vice versa (Fig. S7S); and NL3 by overlap with NL2 clusters (yellow arrows) and wider distribution than NL2 (white arrows) in the striatum (Fig. S7T).
Immunoblot.
Whole brains were homogenized using a Potter homogenizer with 15 strokes at 1,000 rpm in 10 volumes of ice-cold homogenizing buffer containing 0.32 M sucrose, 1 mM EDTA, 1 mM EGTA, 10 mM Tris⋅HCl (pH 7.2), and 0.4 mM phenylmethylsulfonyl fluoride. Homogenized samples were centrifuged at 1,000 × g for 10 min to remove nuclear fractions. To prepare lysates of transfected cells, HEK293T cells (2 × 106) were transfected with mammalian expression vectors using the calcium phosphate precipitation method, harvested 24–48 h after transfection, and sonicated in 10 volumes of ice-cold homogenizing buffer. Mammalian expression plasmid vectors pCAG-HA-Nrxn1α, pCAG-HA-Nrxn2α, and pCAG-HA-Nrxn3α were kind gifts from Kensuke Futai (University of Massachusetts Medical School, Worcester, MA). NL1–4, GABAARα1, and DARPP32 cDNAs were amplified from a mouse cDNA library by PCR, subcloned into pTracer-GFP (Life Technologies), and sequenced. The lysates were denatured in 50 mM (±)-DTT at 65 °C for 30 min. Proteins were separated by SDS/PAGE and electroblotted onto transfer membranes (Immobilon-P; Millipore). Membranes were successively incubated with 5% (wt/vol) skimmed milk for 30 min, primary antibody (1 μg/mL) for 1 h, and peroxidase-linked secondary antibody (1:10,000; Jackson ImmunoResearch) for 1 h. Tris-buffered saline (pH 7.5) containing 0.1% Tween 20 was used as a diluent and washing buffer. Proteins were visualized with ECL prime Western blotting detection reagent (GE Healthcare).
Immunofluorescence.
All incubations were performed at room temperature. Sections were incubated successively with 10% (vol/vol) normal donkey serum for 20 min, a mixture of primary antibodies overnight (1 µg/mL), and a mixture of species-specific secondary antibodies for 2 h at a dilution of 1:200 (Life Technologies; Jackson ImmunoResearch; Abcam; Biotium). Combinations of primary and secondary antibodies for multiple immunofluorescence are listed in Table S2. For the detection of GABAARα1, microslicer sections were subjected to antigen exposure pretreatment before normal donkey serum blocking: 1 mg/mL pepsin in 0.2 N HCl at 37 °C for 30–60 s. PBS containing 0.1% Tween 20 was used as a dilution and washing buffer. Images were taken with a confocal laser-scanning microscope equipped with 405-, 473-, 559-, and 647-nm diode laser lines and UPLSAPO 10× (NA, 0.4), PLAPON 60×OSC2 (NA, 1.4; oil immersion), and UPLSAPO 100×S (NA, 1.35; silicone oil immersion) objective lenses (FV1200, Olympus) under the following microscopic settings: zoom factor, 1, 4, 6, and 10; image size, 640 × 640 pixels; pinhole size, 1 airy unit. Figs. S1H and S2 G–I were taken with an FV-OSR software module (Olympus) to obtain superresolution images. To obtain high-resolution images shown in Fig. 1 A and B, and Figs. S4A and S7 A–C, a series of images were taken with a UPLSAPO 10× objective lens (zoom facter 1.3, 256 × 256 pixels) and were stitched using FV10-ASW 3.0 (Olympus).
Table S2.
List of antibodies used for immunohistochemical data
| Figure | First antibodies (species) | Second antibodies (company) |
| 1A | TH (Rb) | Cy3 (Jackson Immunoresearch) |
| 1B | DAT (Rb) | Cy3 (Jackson Immunoresearch) |
| 1C | DAT (GP) | A488 (Jackson Immunoresearch) |
| VMAT2 (Rb) | Cy3 (Jackson Immunoresearch) | |
| TH (Ms) | A647 (Life Technologies) | |
| 3A | DAT (GP) | A488 (Jackson Immunoresearch) |
| GABAARα1 (Rb) | Cy3 (Jackson Immunoresearch) | |
| VIAAT (Go) | A647 (Life Technologies) | |
| 3B | DAT (GP) | A488 (Jackson Immunoresearch) |
| Gephyrin (Ms) | Cy3 (Jackson Immunoresearch) | |
| VIAAT (Go) | A647 (Life Technologies) | |
| 3C | DAT (GP) | A488 (Jackson Immunoresearch) |
| NL2 (Rb) | Cy3 (Jackson Immunoresearch) | |
| VIAAT (Go) | A647 (Life Technologies) | |
| 4B | D1R (GP) | CF405S (Biotium) |
| GFP (Go) | A488 (Life Technologies) | |
| NL2 (Rb) | Cy3 (Jackson Immunoresearch) | |
| TH (Ms) | A647 (Life Technologies) | |
| 4C | A2AR (GP) | CF405S (Biotium) |
| GFP (Go) | A488 (Life Technologies) | |
| NL2 (Rb) | Cy3 (Jackson Immunoresearch) | |
| TH (Ms) | A647 (Life Technologies) | |
| 5B | NL2 (Rb) | A488 (Life Technologies) |
| DAT (Go) | Cy3 (Jackson Immunoresearch) | |
| CAST (GP) | A647 (Jackson Immunoresearch) | |
| 5C | GABAARα1 (Rb) | A488 (Life Technologies) |
| DAT (Go) | Cy3 (Jackson Immunoresearch) | |
| CAST (GP) | A647 (Jackson Immunoresearch) | |
| 5D | GFP (Rb) | A488 (Life Technologies) |
| DAT (Go) | Cy3 (Jackson Immunoresearch) | |
| CAST (GP) | A647 (Jackson Immunoresearch) | |
| 5F | NL2 (GP) | A488 (Jackson Immunoresearch) |
| DAT (Go) | Cy3 (Jackson Immunoresearch) | |
| VMAT2 (Rb) | A647 (Life Technologies) | |
| 5G | NL2 (GP) | A488 (Jackson Immunoresearch) |
| DAT (Go) | Cy3 (Jackson Immunoresearch) | |
| Nrxn (Rb) | A647 (Life Technologies) | |
| 6 A–C | VIAAT (Go) | A405 (Abcam) |
| GFP (GP) | A488 (Jackson Immunoresearch) | |
| TH (Rb) | Cy3 (Jackson Immunoresearch) | |
| Gephyrin (Ms) | A647 (Life Technologies) | |
| S1A | DAT (Go) | A488 (Life Technologies) |
| VGluT1 (Rb) | Cy3 (Jackson Immunoresearch) | |
| S1B | DAT (Go) | A488 (Life Technologies) |
| VGluT2 (GP) | Cy3 (Jackson Immunoresearch) | |
| S1C | DAT (Go) | A488 (Life Technologies) |
| VGluT3 (GP) | Cy3 (Jackson Immunoresearch) | |
| S1D | DAT (Go) | A488 (Life Technologies) |
| GAD (Rb) | Cy3 (Jackson Immunoresearch) | |
| S1E | DAT (Go) | A488 (Life Technologies) |
| VIAAT (Rb) | Cy3 (Jackson Immunoresearch) | |
| S1F | DAT (Go) | A488 (Life Technologies) |
| GAT1 (Rb) | Cy3 (Jackson Immunoresearch) | |
| S1G | DAT (Go) | A488 (Life Technologies) |
| GAT1 (Rb) | Cy3 (Jackson Immunoresearch) | |
| VIAAT (GP) | A647 (Jackson Immunoresearch) | |
| S1H | DAT (Go) | A488 (Life Technologies) |
| CAST (GP) | Cy3 (Jackson Immunoresearch) | |
| S2B | D1R (GP) | A488 (Jackson Immunoresearch) |
| D2R (Rb) | Cy3 (Jackson Immunoresearch) | |
| S2C | MAP2 (Ms) | A405 (Abcam) |
| DARPP32 (GP) | A488 (Jackson Immunoresearch) | |
| D2R (Rb) | Cy3 (Jackson Immunoresearch) | |
| D1R (Go) | A647 (Life Technologies) | |
| S2D | MAP2 (Ms) | A405 (Abcam) |
| PV (GP) | A488 (Jackson Immunoresearch) | |
| D2R (Rb) | Cy3 (Jackson Immunoresearch) | |
| D1R (Go) | A647 (Life Technologies) | |
| S2E | MAP2 (Ms) | A405 (Abcam) |
| nNOS (GP) | A488 (Jackson Immunoresearch) | |
| D2R (Rb) | Cy3 (Jackson Immunoresearch) | |
| D1R (Go) | A647 (Life Technologies) | |
| S2F | MAP2 (Ms) | A405 (Abcam) |
| CHT (GP) | A488 (Jackson Immunoresearch) | |
| D2R (Rb) | Cy3 (Jackson Immunoresearch) | |
| D1R (Go) | A647 (Life Technologies) | |
| S2G | Tau (Ms) | A488 (Life Technologies) |
| D1R (GP) | Cy3 (Jackson Immunoresearch) | |
| DARPP32 (Rb) | A647 (Life Technologies) | |
| S2H | Tau (Ms) | A488 (Life Technologies) |
| D2R (Rb) | Cy3 (Jackson Immunoresearch) | |
| CHT (GP) | A647 (Jackson Immunoresearch) | |
| S2I | Tau (Ms) | A488 (Life Technologies) |
| D2R (Rb) | Cy3 (Jackson Immunoresearch) | |
| DAT (Go) | A647 (Life Technologies) | |
| S4 A and B | GFP (Rb) | A488 (Life Technologies) |
| S4C | PV (GP) | CF405S (Biotium) |
| GFP (Go) | A488 (Life Technologies) | |
| NL2 (Rb) | Cy3 (Jackson Immunoresearch) | |
| TH (Ms) | A647 (Life Technologies) | |
| S4D | nNOS (GP) | CF405S (Biotium) |
| GFP (Go) | A488 (Life Technologies) | |
| NL2 (Rb) | Cy3 (Jackson Immunoresearch) | |
| TH (Ms) | A647 (Life Technologies) | |
| S4E | CHT (GP) | CF405S (Biotium) |
| GFP (Go) | A488 (Life Technologies) | |
| NL2 (Rb) | Cy3 (Jackson Immunoresearch) | |
| TH (Ms) | A647 (Life Technologies) | |
| S5B | NL2 (Rb) | A488 (Life Technologies) |
| Tau (Ms) | Cy3 (Jackson Immunoresearch) | |
| CAST (GP) | A647 (Jackson Immunoresearch) | |
| S5C | GABAARα1 (Rb) | A488 (Life Technologies) |
| Tau (Ms) | Cy3 (Jackson Immunoresearch) | |
| CAST (GP) | A647 (Jackson Immunoresearch) | |
| S5D | GFP (Rb) | A488 (Life Technologies) |
| Tau (Ms) | Cy3 (Jackson Immunoresearch) | |
| CAST (GP) | A647 (Jackson Immunoresearch) | |
| S5 I and J | VMAT2 (Rb) | Cy3 (Jackson Immunoresearch) |
| S5 N and O | Gephyrin (Ms) | Cy3 (Jackson Immunoresearch) |
| S5 S and T | NL3 (GP) | Cy3 (Jackson Immunoresearch) |
| S5X | NL3 (Rb) | A488 (Life Technologies) |
| Tau (Ms) | Cy3 (Jackson Immunoresearch) | |
| CAST (GP) | A647 (Jackson Immunoresearch) | |
| S6C | GFP (Rb) | A488 (Life Technologies) |
| DARPP32 (GP) | Cy3 (Jackson Immunoresearch) | |
| S6 D–F | GFP (Go) | A488 (Life Technologies) |
| Gephyrin (Ms) | Cy3 (Jackson Immunoresearch) | |
| NL2 (Rb) | A647 (Life Technologies) | |
| S6 J–L | GFP (Go) | A488 (Life Technologies) |
| Gephyrin (Ms) | Cy3 (Jackson Immunoresearch) | |
| NL3 (GP) | A647 (Jackson Immunoresearch) | |
| S7A | VMAT2 (Rb) | Cy3 (Jackson Immunoresearch) |
| S7B | GAD (Rb) | Cy3 (Jackson Immunoresearch) |
| S7C | VIAAT (Rb) | Cy3 (Jackson Immunoresearch) |
| S7 E–F | Synaptophysin (Rb) | A488 (Life Technologies) |
| CAST (GP) | Cy3 (Jackson Immunoresearch) | |
| S7 I and J | D1R (GP or Go) | Cy3 (Jackson Immunoresearch) |
| S7 K and L | D2R (Rb) | Cy3 (Jackson Immunoresearch) |
| S7O | DARPP32 (GP or Rb) | Cy3 (Jackson Immunoresearch) |
| S7P | PV (Rb) | A488 (Life Technologies) |
| DARPP32 (GP) | Cy3 (Jackson Immunoresearch) | |
| S7Q | CHT (Rb) | A488 (Life Technologies) |
| DARPP32 (GP) | Cy3 (Jackson Immunoresearch) | |
| S7S | NL2 (Rb) | A488 (Life Technologies) |
| Gephyrin (Ms) | Cy3 (Jackson Immunoresearch) | |
| S7T | NL2 (Rb) | A488 (Life Technologies) |
| NL3 (GP) | Cy3 (Jackson Immunoresearch) | |
| DAT (Go) | A647 (Life Technologies) |
A405, Alexa405; A488, Alexa488; A647, Alexa647; Go, goat; GP, guinea pig; Ms, mouse; Rb, rabbit.
Quantitative Assessment of Dopamine and GABAergic Synapses on GFP-Labeled Dendrites.
To assess the density and molecular expression of dopamine and GABAergic synapses on dendrites of given neuronal types, we labeled single neurons with GFP by injecting lentiviral vector to the striatum of mice at 4 wk of age under chloral hydrate anesthesia (350 mg/kg of body weight, i.p.). The lentiviral vector system with murine stem cell virus promoter (MSCV) was kindly provided by St. Jude Children’s Research Hospital. The original lentivector pCL20c-MSCV-GFP was modified by adding the woodchuck hepatitis posttranscriptional regulatory element (WPRE) sequence downstream of GFP to enhance GFP expression. Injected mice were fixed 1–2 mo later by transcardial perfusion of fixative and processed for multiple immunofluorescence.
We randomly selected GFP-labeled dendrites that ran near and parallel to the section surface, and obtained their z-stacked image series consisting of 20–50 optical sections in steps of 0.2 µm with PLAPON 60×OSC2 and a zoom factor of 6 or 10 for 3D-reconstruction with Imaris (Bitplane) or ImageJ (imagej.nih.gov) software. Dopamine and GABAergic synapses were defined as NL2+ or gephyrin+ clusters on GFP-labeled dendrites in contact with TH-labeled dopaminergic terminals or VIAAT-labeled GABAergic terminals, respectively. Apposition of the three fluorescences was confirmed by images of the X–Y, X–Z, and Y–Z planes. For quantification, we sampled 5–10 dendrites (>10 µm in length and 0.75–1.5 µm in diameter) from each of three mice in each group. The mean length (µm) and the mean diameter (µm) of analyzed dendrites (mean ± SEM) were as follows: 17.7 ± 1.4 and 1.08 ± 0.03 for d-MSNs (Fig. 4B), 18.0 ± 1.4 and 1.13 ± 0.04 for i-MSNs (Fig. 4C), 17.5 ± 0.87 and 1.09 ± 0.06 for PV+ interneurons (Fig. S4C), 18.9 ± 2.1 and 0.98 ± 0.04 for nNOS+ interneurons (Fig. S4D), 15.1 ± 2.0 and 1.10 ± 0.06 for CHT+ interneurons (Fig. S4E), 20.1 ± 1.2 and 1.04 ± 0.03 for control neurons (Fig. 6A and Fig. S6 D and J), 17.5 ± 2.8 and 0.96 ± 0.03 for NL2-KD#1 neurons (Fig. 6B and Fig. S6 E and K), and 21.8 ± 2.8 and 1.02 ± 0.03 for NL2-KD#2 neurons (Fig. 6C and Fig. S6 F and L). Dendritic spines were also sampled from six spiny dendrites from each of three mice. The mean length (µm) of dendritic spines was 1.45 ± 0.03 for d-MSNs and 1.52 ± 0.03 for i-MSNs (Fig. 4D, filled columns). The density of synapses was shown as mean ± SEM of synapse numbers per 10 µm of dendritic shafts or spines. Fluorescent intensities for gephyrin, NL2, and NL3 clusters on GFP-labeled dendritic shafts (mean ± SEM) were measured from three to four dendrites in each of three mice, and the background level, as estimated from those in the nucleus, was subtracted (Fig. S6 H, I, and M). All of the images presented in this study were taken as single optical sections.
Criteria of Synapses.
Synapses were identified by electron microscopy as interneuronal junctions with the following specializations: rigid and parallel apposition of the apposed membranes, the extracellular space stuffed with some electron-dense materials, accumulation of synaptic vesicles and fuzzy active zone in presynaptic elements, and attachment of the postsynaptic density (PSD) to the cytoplasmic side of the postsynaptic membrane. Asymmetric synapses were defined as synapses whose postsynaptic membrane is attached by thick PSD, by which the postsynaptic specialization appeared more dense than the presynaptic specialization (1). In comparison, symmetrical synapses were defined as those having pale and thin PSD, with almost comparable thickness between the postsynaptic and presynaptic specializations.
By postembedding immunogold electron microscopy, we identified dopamine synapses as TH-labeled symmetric synapses, GABAergic synapses as VIAAT-labeled symmetrical synapses, and glutamatergic synapses as asymmetrical synapses without TH or VIAAT labeling. To minimize the sampling of false-positive dopamine and GABAergic synapses, we only analyzed symmetric synapses having five or more metal particles for TH or VIAAT, respectively, on the presynaptic cytoplasm excluding the mitochondria. The mean and SEM of data obtained from postembedding immunogold analysis were calculated from the mean values of three mice in each group, as shown in Table S3.
Pre-Embedding Immunoelectron Microscopy.
For single-label pre-embedding immunogold electron microscopy for D1R (Fig. 2A), D2R (Fig. 2B), DAT (Fig. S5 F and G), and VMAT2 (Fig. S5 K and L), microslicer sections were dipped in Blocking Solutions (Aurion) for 30 min, and incubated overnight with primary antibodies to D1R (guinea pig), D2R (rabbit), DAT (rabbit), and VMAT2 (rabbit) (1 µg/mL) diluted with 1% BSA/0.004% saponin in PBS and then with secondary antibodies linked to 1.4-nm gold particles (1:100; Nanogold; Nanoprobes) for 2 h. After postfixaion with 1% glutaraldehyde in PBS for 15 min, immunogold was intensified with a silver enhancement kit (R-GENT SE-EM; Aurion) for 30–60 min.
For double-label pre-embedding immunoelectron microscopy (Fig. 2 D and E), sections labeled for guinea pig D1R or D2R antibody (metal particles) were further incubated for TH labeling. After blocking with 10% (vol/vol) normal guinea pig serum for 30 min, sections were incubated for rabbit TH antibody (1 µg/mL) overnight, biotin-conjugated donkey anti-rabbit secondary antibody (1:200; Jackson ImmunoResearch) for 2 h, and peroxidase-conjugated streptoavidin (Nichirei) for 30 min. TH labeling was visualized as 3,3′-diaminobenzidine (DAB) precipitates with a DAB substrate kit (SK-4100; Vector). Immunolabeled sections were treated with 1% osmium tetroxide for 15 min, block-stained with 2% (wt/vol) uranyl acetate for 20 min, dehydrated with a graded ethanol series and propylene oxide twice for 10 min each, and embedded in Epon 812 (TAAB). Ultrathin sections were prepared with an ultramicrotome (Ultracut; Leica) and mounted on copper-mesh grids.
Quantitative Measurement for Pre-Embedding Immunoelectron Microscopy.
To avoid uneven antibody penetration, electron micrographs were taken within 5 µm (D1R or D2R in Fig. 2 A, B, D, and E; VMAT2 in Fig. S5 K and L) or 10 µm (DAT in Fig. S5 F and G) from the surface of sections subjected to pre-embedding immunoelectron microscopy. To quantify metal particle labeling for D1R or D2R (Fig. 2C), 3 × 4 montage images (∼3.6 µm × 4.8 µm) were randomly taken at a magnification of 20,000×. In double-label pre-embedding immunoelectron microscopy for TH and D1R or D2R (Fig. 2G), we searched dendrites, whose profiles formed TH-labeled dopamine synapses and had three or more metal particles for D1R or D2R, at a magnification of 4,000×. Then, 3 × 3 montage images (∼3.6 µm × 3.6 µm) over dopamine synapses were taken at a magnification of 20,000×, and four to seven dopamine synapses were sampled from each of three mice in each group. The density of labeling was calculated per 1 µm of the synaptic, perisynaptic, and extrasynaptic membranes.
Metal particles for VMAT2 were also measured at dopamine synapses as the density per 1 µm2 of the cytoplasm (Fig. S5M) by sampling nerve terminals, which contained five or more particles for VMAT2 and formed symmetric synapses with dendritic shafts and spines. We took 3 × 3 montage images (∼3.6 µm × 3.6 µm) at a magnification of 20,000×, with five to eight terminals from each of three mice in each group.
Postembedding Immunoelectron Microscopy.
Double-label postembedding immunogold electron microscopy was used to quantify molecular expression at TH-labeled dopamine synapses (Figs. 1 E, G, and I and 3 D–F and Figs. S2 J and K, S3 D and E, and S5 P, Q, U, and V), at VIAAT-labeled GABAergic synapses (Fig. 1D and Figs. S1 I and K and S3 A–C), or glutamatergic synapses without TH or VIAAT labeling (Fig. 1D and Figs. S1 J and L and S3 D and E). Ultrathin sections on nickel grids were successively treated with the following solutions: saturated sodium ethoxide for 2 s, 50 mM glycine in incubation solution (0.03% Triton X-100 in Tris-buffered saline, pH 7.4; TBST) for 10 min, blocking solution containing 2% (vol/vol) normal goat serum in TBST (GTBST) for 10 min, primary antibodies (1:1,000 dilution for GABA antibody and 20 μg/mL for CAST D1R, D2R, GABAARα1, gephyrin, NL2, NL3, Nrxn pan-AMPAR, PSD-95) diluted in GTBST overnight, and colloidal gold-conjugated (10-nm diameter) secondary antibodies (1:100; British BioCell International) in GTBST for 2 h. After blocking with 2% (vol/vol) normal rabbit or guinea pig serum in TBST, sections were subjected successively to mouse or rabbit TH antibody or rabbit or guinea pig VIAAT antibody in GTBST (20 or 10 µg/mL, respectively) for 6 h and colloidal gold-conjugated (15-nm diameter) secondary antibodies (1:100; British BioCell International) in GTBST for 2 h. Sections were washed thoroughly in distilled water, and stained with 5% (wt/vol) uranyl acetate in 40% (vol/vol) EtOH for 90 s and Reynold’s lead citrate solution for 90 s Photographs were taken with a JEM1400 electron microscope (JEOL).
Quantitative Measurement for Postembedding Immunoelectron Microscopy.
First we searched asymmetrical synapses or symmetrical synapses with immunogold labeling for TH or VIAAT at a magnification of 5,000×, and took 3 × 3 montage images (∼2.4 µm × 2.4 µm) of glutamatergic, dopamine, and GABAergic synapses at a magnification of 30,000×. To measure the density of presynaptic labeling for Nrxn (Fig. 1J) and postsynaptic labeling for GABAARα1, gephyrin, NL2, NL3, PSD-95, and AMPAR (Fig. 3 G–I and Figs. S3 F and G and S5 R and W), immunogold particles <20 nm apart from the center of the cell membrane were measured. In assessment of presynaptic labeling for CAST (Fig. 1H), we counted immunogold particles <40 nm from the center of the presynaptic membrane. We examined 5–15 dopamine, GABAergic, or glutamatergic synapses from each of three mice in each group, with the number of analyzed synapses ranging fewer than seven synapses in the same groups. Metal particles for GABA were also measured at dopamine, GABAergic, or glutamatergic synapses as the density per 1 µm2 of the cytoplasm excluding the mitochondria (Fig. 1F).
The density of D1R and D2R labelings on the synaptic, perisynaptic, and extrasynapti membranes at around dopamine synapses (Fig. S2 L and M) was calculated by measuring immunogold particles <20 nm apart from the center of the cell membrane in contact with TH-labeled dopamine terminals. From each of three mice, 17–20 dendritic profiles forming dopamine synapses were analyzed. The density of immunogold labeling at given synapse types or compartments was calculated as that per 1 µm of the synaptic, perisynaptic, and extrasynaptic membranes.
Dopamine Synapse Density.
The density of dopamine synapses in the striatum was compared between NL2-KO and wild-type mice by measuring the nearest neighbor distance (Fig. S5H). Dopamine synapses, which were labeled with five or more metal particles for DAT and formed symmetric synapses on dendritic shafts and spines, were sampled, and their 5 × 5 montage images (∼11 µm × 11 µm) were taken at a magnification of 10,000×. We measured distances from the center of the postsynaptic membrane of given dopamine synapses to that of the nearest ones, and the mean nearest neighbor distance was calculated by sampling five to eight dopamine synapses from each of three mice in each group.
Knockdown by MicroRNA.
For miR-based knockdown, we used the BLOCK-iT Pol II miR RNAi Expression Vector Kits (Life Technologies) according to the manufacturer’s instructions. pCL20c-MSCV-GFP-miR-WPRE was obtained by inserting the following miR sequences into pCL20c-MSCV-GFP-WPRE: 5′-GTGTACATCCTGGTCCACTAGCGTTTTGGCCACTGACTGACGCTAGTGGCAGGATGTACAC-3′ (NL2-miR#1); 5′-GTGAACTTGGTGTCCTGTGGCAGTTTTGGCCACTGACTGACTGCCACAGCACCAAGTTCA-3′ (NL2-miR#2); 5′-GAAATGTACTGCGCGTGGAGACGTTTTGGCCACTGACTGACGTCTCCACGCAGTACATTT-3′ (control-miR).
Lentiviral vectors were produced by cotransfection of HEK293T cells (5 × 106 per one dish) with a mixture of pCL20c-MSCV-GFP-WPRE or pCL20c-MSCV-GFP-miR-WPRE (20 µg per 1 dish), pCAG-kGP4.1R (12 µg), pCAG4-RTR2 (4 µg), and pCAGGS-VSVG (4 µg) using the calcium phosphate precipitation method. HEK293T cells were incubated in DMEM (D5796; Sigma) supplemented with 10% (vol/vol) FBS (Cell Culture Bioscience), 1× penicillin/streptomycin (P4333; Sigma), 1 mM sodium pyruvate (S8636; Sigma), 1× MEM nonessential amino acid solution (M7145; Sigma), and 1× GlutaMAX (#35050061; Life Technologies) at 37 °C in a 10% CO2 atmosphere. Culture medium was replaced with Ultraculture (12-725F, Lonza) 16 h after transfection. Supernatant containing viral particles was collected 40 h after transfection, centrifuged at 1,000 × g for 10 min, filtered through 0.45-μm membranes, and concentrated using an Amicon Ultra filter unit 100k (Millipore). Viral solution was further concentrated by centrifuging at 6,000 × g for 16 h, before suspending in PBS. Viral titer [expressed as transducing units (TU)] was calculated as 1.0 × 108–9 TU/mL by measuring the number of infected HEK293T cells.
For viral injection in newborn pups, deep anesthesia was obtained by lowering the body temperature on ice, and the skull was fastened with a handmade stereotaxic instrument. A glass pipette (G-1.2; Narishige) filled with 0.5 μL of viral solution was inserted into the striatum. Viral solutions were injected by air pressure (Pneumatic Picopump; World Precision Instruments). Injected mice were fixed 2 mo later by transcardial perfusion of fixative, as described above.
Coculture Assay.
Coculture experiments were performed according to previous methods with modifications (32). Briefly, the midbrain and striatum from newborn mice were collected in ice-cold Hank’s balanced solution (HBS), and dissociated in 0.25% trypsin/HBS for 10 min and 0.25% trypsin/0.1% DNaseI/HBS for 5 min at 37 °C. Dissociated neurons were suspended in neuronal basal medium supplemented with B-27 solution (both from Life Technologies) and GlutaMAX, seeded on plastic dishes coated with 5% (vol/vol) Matrigel (BD Biosciences) at a density of 1–3 × 104 cells/cm2, and incubated at 5% CO2. After 9–11 d in vitro, HEK293T cells transfected with pTracer-NL1, pTracer-NL2, pTracer-NL3, pTracer-GABAARα1, and pEGFP-N1 (Clontech) were seeded on the primary midbrain or striatal culture at a density of 2 × 104 cells/cm2. After 48 h, cells were fixed with 4% (wt/vol) paraformaldehyde in 0.1 M PB for 10 min at room temperature for immunofluorescence.
Statistical Analysis.
For quantitative comparison, immunofluorescence data from three mice in each group were pooled together and are presented as the mean ± SEM. Statistical significance was assessed by Mann–Whitney u test (for comparison of two independent samples) or one-way ANOVA with Tukey’s post hoc test (for comparison of more than three independent samples). Statistical significance of quantitative electron microscopic data were assessed by unpaired t test, because no significant differences were found in variances between two independent samples by F-test.
Acknowledgments
We thank Dr. Kensuke Futai (University of Massachusetts Medical School) for his kind gift of plasmid vectors encoding Nrxn1α–3α cDNA, and St. Jude Children’s Research Hospital for providing the lentiviral vector system. This study was supported by Grants-in-Aid for Scientific Research 24220007 (to M.W.) and 15K06732 (to M.U.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1514074113/-/DCSupplemental.
References
- 1.Gray EG. Axo-somatic and axo-dendritic synapses of the cerebral cortex: An electron microscope study. J Anat. 1959;93:420–433. [PMC free article] [PubMed] [Google Scholar]
- 2.Kuzirian MS, Paradis S. Emerging themes in GABAergic synapse development. Prog Neurobiol. 2011;95(1):68–87. doi: 10.1016/j.pneurobio.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lardi-Studler B, Fritschy JM. Matching of pre- and postsynaptic specializations during synaptogenesis. Neuroscientist. 2007;13(2):115–126. doi: 10.1177/1073858406296803. [DOI] [PubMed] [Google Scholar]
- 4.Spitzer NC, Borodinsky LN. Implications of activity-dependent neurotransmitter-receptor matching. Philos Trans R Soc Lond B Biol Sci. 2008;363(1495):1393–1399. doi: 10.1098/rstb.2007.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams ME, de Wit J, Ghosh A. Molecular mechanisms of synaptic specificity in developing neural circuits. Neuron. 2010;68(1):9–18. doi: 10.1016/j.neuron.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Südhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455(7215):903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song JY, Ichtchenko K, Südhof TC, Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci USA. 1999;96(3):1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varoqueaux F, Jamain S, Brose N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol. 2004;83(9):449–456. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- 9.Chubykin AA, et al. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54(6):919–931. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- 12.Freund TF, Powell JF, Smith AD. Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience. 1984;13(4):1189–1215. doi: 10.1016/0306-4522(84)90294-x. [DOI] [PubMed] [Google Scholar]
- 13.Pickel VM, Beckley SC, Joh TH, Reis DJ. Ultrastructural immunocytochemical localization of tyrosine hydroxylase in the neostriatum. Brain Res. 1981;225(2):373–385. doi: 10.1016/0006-8993(81)90843-x. [DOI] [PubMed] [Google Scholar]
- 14.Moss J, Bolam JP. A dopaminergic axon lattice in the striatum and its relationship with cortical and thalamic terminals. J Neurosci. 2008;28(44):11221–11230. doi: 10.1523/JNEUROSCI.2780-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caillé I, Dumartin B, Bloch B. Ultrastructural localization of D1 dopamine receptor immunoreactivity in rat striatonigral neurons and its relation with dopaminergic innervation. Brain Res. 1996;730(1-2):17–31. doi: 10.1016/0006-8993(96)00424-6. [DOI] [PubMed] [Google Scholar]
- 16.Sesack SR, Aoki C, Pickel VM. Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. J Neurosci. 1994;14(1):88–106. doi: 10.1523/JNEUROSCI.14-01-00088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yung KK, et al. Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: Light and electron microscopy. Neuroscience. 1995;65(3):709–730. doi: 10.1016/0306-4522(94)00536-e. [DOI] [PubMed] [Google Scholar]
- 18.Stensrud MJ, Puchades M, Gundersen V. GABA is localized in dopaminergic synaptic vesicles in the rodent striatum. Brain Struct Funct. 2014;219(6):1901–1912. doi: 10.1007/s00429-013-0609-4. [DOI] [PubMed] [Google Scholar]
- 19.Arluison M, de la Manche IS. High-resolution radioautographic study of the serotonin innervation of the rat corpus striatum after intraventricular administration of [3H]5-hydroxytryptamine. Neuroscience. 1980;5(2):229–240. doi: 10.1016/0306-4522(80)90100-1. [DOI] [PubMed] [Google Scholar]
- 20.Dong N, Qi J, Chen G. Molecular reconstitution of functional GABAergic synapses with expression of neuroligin-2 and GABAA receptors. Mol Cell Neurosci. 2007;35(1):14–23. doi: 10.1016/j.mcn.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Tyagarajan SK, Fritschy JM. Gephyrin: A master regulator of neuronal function? Nat Rev Neurosci. 2014;15(3):141–156. doi: 10.1038/nrn3670. [DOI] [PubMed] [Google Scholar]
- 22.Quiroz C, et al. Key modulatory role of presynaptic adenosine A2A receptors in cortical neurotransmission to the striatal direct pathway. ScientificWorldJournal. 2009;9:1321–1344. doi: 10.1100/tsw.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon HB, et al. Neuroligin-1-dependent competition regulates cortical synaptogenesis and synapse number. Nat Neurosci. 2012;15(12):1667–1674. doi: 10.1038/nn.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pereira DB, Sulzer D. Mechanisms of dopamine quantal size regulation. Front Biosci (Landmark Ed) 2012;17:2740–2767. doi: 10.2741/4083. [DOI] [PubMed] [Google Scholar]
- 25.Ohtsuka T. CAST: Functional scaffold for the integrity of the presynaptic active zone. Neurosci Res. 2013;76(1-2):10–15. doi: 10.1016/j.neures.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Fujiyama F, Fritschy JM, Stephenson FA, Bolam JP. Synaptic localization of GABA(A) receptor subunits in the striatum of the rat. J Comp Neurol. 2000;416(2):158–172. [PubMed] [Google Scholar]
- 27.El Mestikawy S, Wallén-Mackenzie A, Fortin GM, Descarries L, Trudeau LE. From glutamate co-release to vesicular synergy: Vesicular glutamate transporters. Nat Rev Neurosci. 2011;12(4):204–216. doi: 10.1038/nrn2969. [DOI] [PubMed] [Google Scholar]
- 28.Tritsch NX, Ding JB, Sabatini BL. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 2012;490(7419):262–266. doi: 10.1038/nature11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanley JJ, Bolam JP. Synaptology of the nigrostriatal projection in relation to the compartmental organization of the neostriatum in the rat. Neuroscience. 1997;81(2):353–370. doi: 10.1016/s0306-4522(97)00212-1. [DOI] [PubMed] [Google Scholar]
- 30.Tritsch NX, Oh WJ, Gu C, Sabatini BL. Midbrain dopamine neurons sustain inhibitory transmission using plasma membrane uptake of GABA, not synthesis. eLife. 2014;3:e01936. doi: 10.7554/eLife.01936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poulopoulos A, et al. Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin. Neuron. 2009;63(5):628–642. doi: 10.1016/j.neuron.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 32.Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101(6):657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 33.Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307(5713):1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- 34.Varoqueaux F, et al. Neuroligins determine synapse maturation and function. Neuron. 2006;51(6):741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Agnati LF, Guidolin D, Guescini M, Genedani S, Fuxe K. Understanding wiring and volume transmission. Brain Res Brain Res Rev. 2010;64(1):137–159. doi: 10.1016/j.brainresrev.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Yamasaki M, Matsui M, Watanabe M. Preferential localization of muscarinic M1 receptor on dendritic shaft and spine of cortical pyramidal cells and its anatomical evidence for volume transmission. J Neurosci. 2010;30(12):4408–4418. doi: 10.1523/JNEUROSCI.5719-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rice ME, Patel JC, Cragg SJ. Dopamine release in the basal ganglia. Neuroscience. 2011;198:112–137. doi: 10.1016/j.neuroscience.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marcott PF, Mamaligas AA, Ford CP. Phasic dopamine release drives rapid activation of striatal D2-receptors. Neuron. 2014;84(1):164–176. doi: 10.1016/j.neuron.2014.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Séguéla P, Watkins KC, Descarries L. Ultrastructural relationships of serotonin axon terminals in the cerebral cortex of the adult rat. J Comp Neurol. 1989;289(1):129–142. doi: 10.1002/cne.902890111. [DOI] [PubMed] [Google Scholar]
- 40.Séguéla P, Watkins KC, Geffard M, Descarries L. Noradrenaline axon terminals in adult rat neocortex: An immunocytochemical analysis in serial thin sections. Neuroscience. 1990;35(2):249–264. doi: 10.1016/0306-4522(90)90079-j. [DOI] [PubMed] [Google Scholar]
- 41.Wainer BH, et al. Cholinergic synapses in the rat brain: A correlated light and electron microscopic immunohistochemical study employing a monoclonal antibody against choline acetyltransferase. Brain Res. 1984;308(1):69–76. doi: 10.1016/0006-8993(84)90918-1. [DOI] [PubMed] [Google Scholar]
- 42.Takács VT, Freund TF, Nyiri G. Neuroligin 2 is expressed in synapses established by cholinergic cells in the mouse brain. PLoS One. 2013;8(9):e72450. doi: 10.1371/journal.pone.0072450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.tom Dieck S, et al. Deletion of the presynaptic scaffold CAST reduces active zone size in rod photoreceptors and impairs visual processing. J Neurosci. 2012;32(35):12192–12203. doi: 10.1523/JNEUROSCI.0752-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fukabori R, et al. Striatal direct pathway modulates response time in execution of visual discrimination. Eur J Neurosci. 2012;35(5):784–797. doi: 10.1111/j.1460-9568.2012.08005.x. [DOI] [PubMed] [Google Scholar]
- 45.Sano H, et al. Conditional ablation of striatal neuronal types containing dopamine D2 receptor disturbs coordination of basal ganglia function. J Neurosci. 2003;23(27):9078–9088. doi: 10.1523/JNEUROSCI.23-27-09078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fukaya M, et al. Abundant distribution of TARP gamma-8 in synaptic and extrasynaptic surface of hippocampal neurons and its major role in AMPA receptor expression on spines and dendrites. Eur J Neurosci. 2006;24(8):2177–2190. doi: 10.1111/j.1460-9568.2006.05081.x. [DOI] [PubMed] [Google Scholar]
- 47.Uchigashima M, et al. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci. 2007;27(14):3663–3676. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamasaki M, et al. Opposing role of NMDA receptor GluN2B and GluN2D in somatosensory development and maturation. J Neurosci. 2014;34(35):11534–11548. doi: 10.1523/JNEUROSCI.1811-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iwakura A, Uchigashima M, Miyazaki T, Yamasaki M, Watanabe M. Lack of molecular-anatomical evidence for GABAergic influence on axon initial segment of cerebellar Purkinje cells by the pinceau formation. J Neurosci. 2012;32(27):9438–9448. doi: 10.1523/JNEUROSCI.1651-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fukaya M, Watanabe M. Improved immunohistochemical detection of postsynaptically located PSD-95/SAP90 protein family by protease section pretreatment: a study in the adult mouse brain. J Comp Neurol. 2000;426(4):572–586. [PubMed] [Google Scholar]
- 51.Miura E, et al. Expression and distribution of JNK/SAPK-associated scaffold protein JSAP1 in developing and adult mouse brain. J Neurochem. 2006;97(5):1431–1446. doi: 10.1111/j.1471-4159.2006.03835.x. [DOI] [PubMed] [Google Scholar]
- 52.Somogyi J, et al. GABAergic basket cells expressing cholecystokinin contain vesicular glutamate transporter type 3 (VGLUT3) in their synaptic terminals in hippocampus and isocortex of the rat. Eur J Neurosci. 2004;19(3):552–569. doi: 10.1111/j.0953-816x.2003.03091.x. [DOI] [PubMed] [Google Scholar]