Abstract
Malignant pericardial effusion (MPE) is a serious complication of several cancers. The most commonly involved solid tumors are lung and breast cancer. MPE can give rise to the clinical picture of cardiac tamponade, a life threatening condition that needs immediate drainage. While simple pericardiocentesis allows resolution of the symptoms, MPE frequently relapses unless further procedures are performed. Prolonged drainage, talcage with antineoplastic agents, or surgical creation of a pleuro-pericardial window are the most commonly suggested ones. They all result in MPE resolution and high rates of long-term control. Patients suitable for further systemic treatments can have a good prognosis irrespective of the pericardial site of disease. We prospectively enrolled patients with cardiac tamponade treated with prolonged drainage associated with Bleomycin administration.
Twenty-two consecutive patients with MPE and associated signs of hemodynamical compromise underwent prolonged drainage and subsequent Bleomycin administration. After injection of 100 mg lidocaine hydrochloride, 10 mg Bleomycin was injected into the pericardial space. The catheter was clumped for 48 h and then reopened. Removal was performed when the drainage volume was <25 mL daily.
Twelve patients (54%) achieved complete response and 9 (41%) a partial response. Only 1 (5%) had a treatment failure and underwent a successful surgical procedure. Acute toxicity was of a low degree and occurred in 7 patients (32%). It consisted mainly in thoracic pain and supraventricular arrhythmia. The 1-year pericardial effusion progression-free survival rate was 74.0% (95% confidence interval [CI]: 51.0–97.3). At a median follow-up of 75 months, a pericardial progression was detected in 4 patients (18%). One- and two-year overall survival rates were 33.9% (95% CI: 13.6–54.2) and 14.5% (95% CI: 0.0–29.5), respectively, with lung cancer patients having a shorter survival than breast cancer patients. The worst prognosis, however, was shown in patients not suitable for systemic treatments, irrespective of the site of the primary tumor.
Prolonged drainage and intrapericardial Bleomycin is a safe and effective treatment, which should be considered as first choice at least in patients suitable for active systemic treatment.
INTRODUCTION
Malignant involvement of the pericardium is detected in 2% to 15% of cases in autopsy studies and in 0.1% in cancer clinical history.1–4 The most common primary tumor involving the pericardium is lung cancer; others include breast and esophageal cancer, lymphoma, and leukemia.5–7 In patients with a known malignancy, the development of a symptomatic malignant pericardial effusion (MPE) has been reported to be associated with a median survival of <4 months,8 although prognosis seems influenced by the site of the primary and its natural history.9,10 The clinical picture of malignant involvement of the pericardium can be that of pericarditis, MPE, cardiac tamponade, or pericardial constriction. Cardiac tamponade is a life-threatening complication if not promptly treated. It occurs when the pressure from pericardial effusion impairs ventricular filling, resulting in ventricular diastolic collapse and decreased cardiac output. Pericardiocentesis with cytological examination of the pericardial fluid should be performed in patients with any pericardial effusion whenever there is a reason to suspect malignancy. For symptomatic patients or those with evidence of hemodynamic compromise, urgent fluid removal is needed to alleviate symptoms and prevent cardiogenic shock.11 Although pericardiocentesis effectively relieves symptoms and improves hemodynamics, prevention of effusion recurrence can be particularly challenging, due to a high frequency of reaccumulation and need for reintervention.12 Rates of recurrence were recently estimated to be in the order of 40% in an extended literature review.13
Thus, initial management of an effusion is usually combined with measures to prevent recurrence, such as surgical formation of a pericardial window and extended fluid drainage with or without pericardial sclerosis.14 Talcage involves instilling a sclerosing agent with the intention of scarring the pericardium to the epicardium. Multiple agents have been studied including Tetracycline and Doxycycline,15 Minocycline,16 Thiotepa,17,18 platinum derivatives,19–21 Mitoxantrone,22 Mitomycin C,23 and Bleomycin.24–26 Success rates (expressed as no reaccumulation at 30 days) range from 70% to 90%.27 While surgical procedures have not been object of direct comparison with drainage, the local administration of Bleomycin was compared with prolonged catheter drainage in the context of a prospective randomized trial in patients with moderate to large pericardial effusion due to lung cancer. The authors reported an increased rate of effusion failure-free survival at 2 months, although the difference did not reach statistical significance.28 Moreover, in a head-to-head comparison with Doxycycline, Bleomycin has been shown to have fewer side effects and to lead to shorter hospitalizations.29 Thus, when talcage is performed, Bleomycin can be considered an active and safe agent. In this study, we evaluated the short- and long-term safety, symptom relief, as well as the long-term benefit, of prolonged drainage in association with intrapericardial instillation of Bleomycin, in patients with cardiac tamponade due to metastatic solid tumors.
METHODS
Consecutive patients with MPE associated with a solid tumor and a life expectancy longer than 30 days were enrolled in the study. Enrollment started on March 2009 and the last patient was registered on June 2015. Patients had clinical signs of cardiac tamponade together with echocardiographic evidence of pericardial effusion associated with criteria of hemodynamical compromise (i.e., diastolic collapse of the right ventricular free wall, and systolic collapse of the right atrial free wall). Among the exclusion criteria were considered: platelet count >50,000 or International normalized ratio >1.5, fever and active infection, severe renal or liver function damage, and hematological malignancy. Performance status was not considered among the selection criteria.
Baseline investigation for all patients included history and physical examination, laboratory panel (blood cell count, coagulative profile, and liver and renal function), complete radiological staging of the cancer (that had to include a chest computed tomography [CT] scan), electrocardiogram (ECG), and trans-thoracic echocardiogram. All patients were admitted to hospital and underwent a simultaneous oncological and cardiological evaluation. Treatments were all performed at 3 institutions (S.Croce e Carle Hospital, Cuneo, U.Parini Hospital, Aosta, SS Antonio e Biagio Hospital, Alessandria, all in Italy).
Following echocardiographic confirmation of MPE, under sterile conditions, a pigtail catheter was inserted percutaneously via a subxiphoid approach into the pericardial sac using the Seldinger technique. After the initial drainage, the catheter was left in the pericardial space and fixed to the skin by sutures. The fluid from drained effusions underwent chemical and cytological examination. After 48-h drainage, if the patient was hemodynamically stable, intrapericardial Bleomycin was administered. To prevent pain, Lidocaine hydrochloride 100 mg was injected intrapericardially. Then, Bleomycin 10 mg, dissolved in 20 mL of normal saline, was administered through the catheter into the pericardial sac in a 5-min bolus administration, followed by further 10 mL normal saline. The catheter was clamped for 2 h, and then reopened and allowed to drain. The catheter was rinsed with normal saline every 12 h and was removed when the net drainage was <25 mL per 24 h and echocardiography showed minimal or no residual effusion. Further Bleomycin administrations were given in case of persistent drainage >25 mL/d and/or evidence of residual pericardial fluid.
Echocardiography was repeated daily until catheter removal. Clinical and echocardiographic controls were programmed at 14 days and then monthly for at least 6 months. Further controls were planned according to the presence of symptoms suggestive of MPE. CT scan was used to evaluate effusion after 6 months. Referral to echocardiography was indicated in case of suggestive symptoms or abnormal findings at the CT scan.
Baseline patient characteristics, treatment features, and outcomes were prospectively recorded until patient death. In case of relapse, a further talcage was deemed possible, although the alternative procedure of a surgical pleuro-pericardial window was also considered. A complete response was defined as no reaccumulation of fluid at the first evaluation (14 days). A partial response was defined as the presence of residual fluid not requiring aspiration within the evaluation period. Patients requiring repeated drainage within 14 days were classified as treatment failures. Pericardial progression-free survival was calculated from the day of Bleomycin administration to the date of MPE relapse, death or last follow-up. Overall survival was calculated from the date of first instillation of Bleomycin to the date of death or last follow-up evaluation. Time-to-event outcomes were accounted using the Kaplan–Meyer method. Comparisons between different samples were made using the univariate log-rank test, and differences were considered statistically significant for P values < 0.05. Treatment toxicity was evaluated through clinical assessment and ECG monitoring until the catheter was in site and was assessed according to the National Cancer Institute common toxicity criteria (NCI-CTC version 13). All the patients signed an informed consent form before undergoing the procedure. The treatment was not considered experimental and submission to the ethical committee was not considered necessary. A formal internal protocol was approved by hospital regulatory departments.
RESULTS
Twenty-two patients with cancer-related cardiac tamponade were enrolled. Baseline characteristics are listed in Table 1. The majority of the patients had nonsmall-cell lung cancer, while 5 patients had breast cancer, 1 patient cervical cancer, 1 mesothelioma, and 1 gastric cancer. Among the 14 patients with lung cancer, histology was adenocarcinoma in 8. Epidermal growth factor receptor (EGFR) mutational status was assessed in only 4 of them and no mutations were detected. In only 2 patients, MPE was the only evident site of disease while in the other 20 MPE was associated with other metastatic sites. Dyspnea was the most common presenting symptom. Characteristics of the technical procedure and of acute events are listed in Table 2. Pericardial fluid was macroscopically bloody in 64% and it was positive for malignant cells in 68%. The median drainage volume was 800 mL (range 500–1350 mL) in accordance with the baseline clinical picture of tamponade.
TABLE 1.
Patient Characteristics
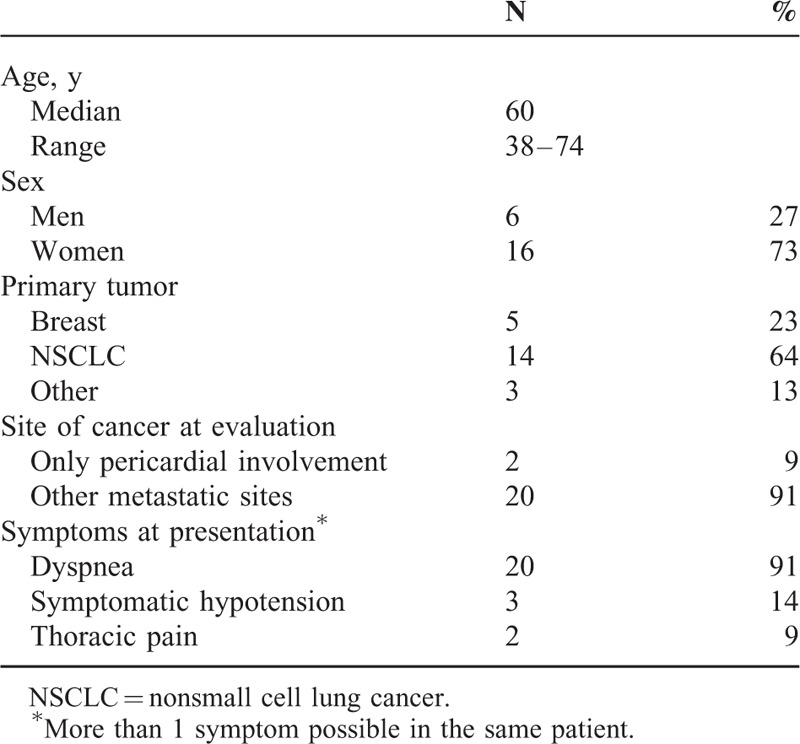
TABLE 2.
Characteristics of the Technical Procedure and Acute Events
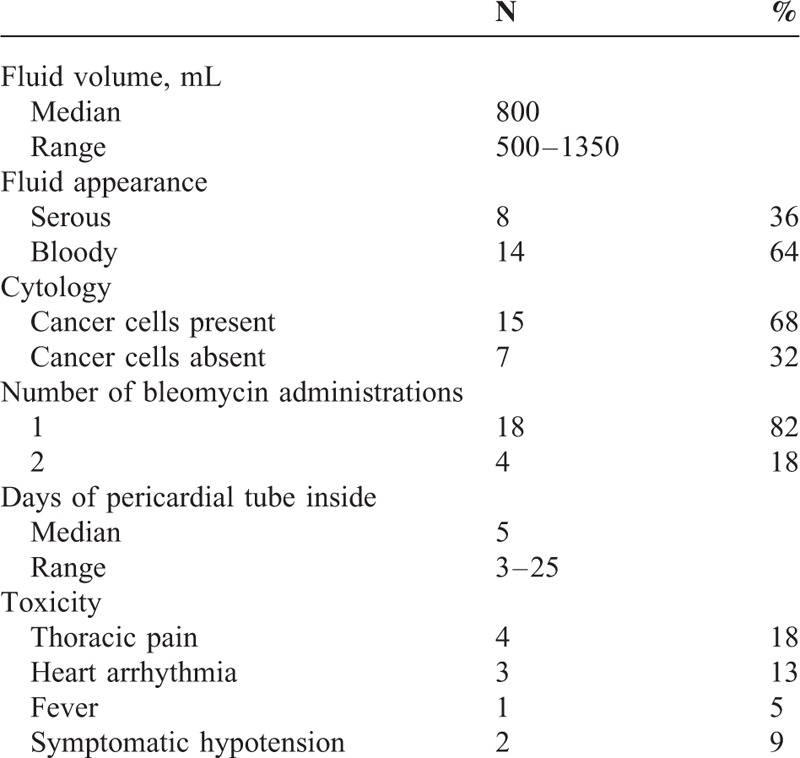
Of the 22 patients, 18 (82%) received a single intrapericardial Bleomycin administration while in 4 patients (18%) the persistence of fluid effusion requested a second talcage procedure. No patient required further procedures. The pericardial tube was removed after a median time of 5 days (range 3–25). All the patients had the tube removed within 7 to 8 days. One patient was referred for Bleomycin administration 12 days after tube placement so that the overall time of drainage reached 25 days. All patients had a sudden relief of symptoms and 15 of them (68%) had no side effects. Some form of acute toxicity occurred in 7 patients (32%) and was never severe. Thoracic pain was the most common occurring event (18%). Arrhythmia was the second more frequent event (13%), while fever and transient hypotension occurred, respectively, in 1 and 2 patients. One patient developed supraventricular tachycardia that requested pharmacological treatment, but in this case MPE was associated with lung cancer-related myocardial infiltration. All the side effects disappeared in few days and in patients undergoing long-term follow-up constrictive pericarditis was never detected.
The echocardiographic evaluation performed at day 14 in 12 cases (54%) showed no sign of residual fluid while in 9 (41%); there was residual fluid without hemodynamic impact. In 1 case, a significant amount of fluid was detected that had hemodynamic impact and requested urgent referral to the cardiothoracic surgeon (treatment failure rate of 5%). A pleuro-pericardial window was performed using a subxifoid approach, with resolution of the pericardial effusion.
Of the 22 treated patients, 12 underwent further active systemic treatment (chemotherapy) while 10 were referred to the palliative care services due to low performance status (7 with lung cancer, 2 with breast cancer, and 1 with cervical cancer) and underwent supportive treatment alone.
At a median follow-up of 75 months, a pericardial progression was detected in 4 patients (19%): 1 was not responder to Bleomycin talcage; the others progressed at 20 days, 40 days, and 5 months, respectively. One patient underwent a second drainage and a successful Bleomycin talcage. The other 3 cases were referred to cardio-thoracic surgery and underwent a surgical pleuro-pericardial window without any further relapse The 12-month pericardial effusion progression-free survival rate was 74.0% (95% confidence interval [CI]: 51.0–97.3; Figure 1). Median time to pericardial effusion progression was not reached.
FIGURE 1.
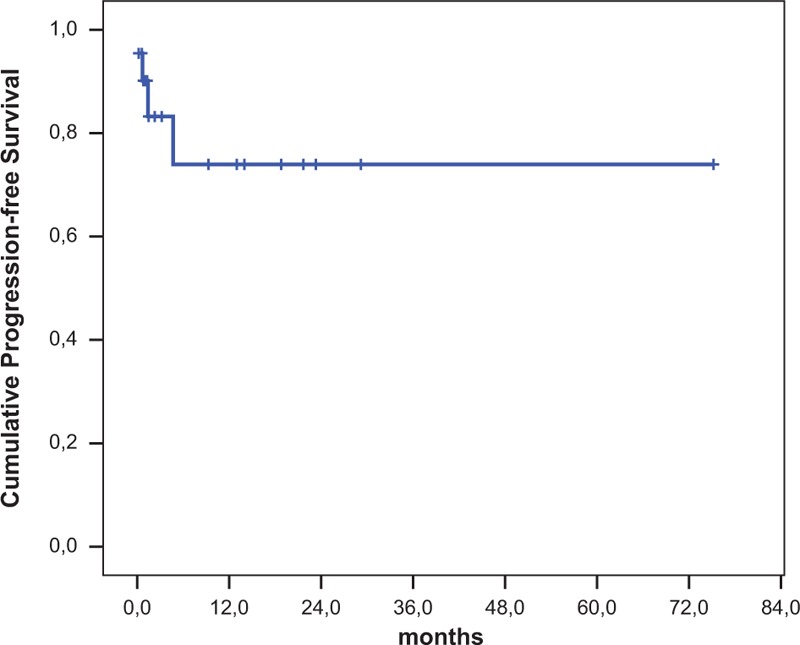
Pericardial progression-free survival.
For all patients who died the cause of death was extra-pericardial disease progression, and no patient died due to pericardial effusion. Seven patients survived more than 1 year from treatment of pericardial effusion: 3 breast cancer and 4 lung cancer patients.
The 6-, 12-, and 24-month overall survival rate was 38.8% (95% CI: 18.1–59.5), 33.9% (95% CI: 13.6–54.2), and 14.5% (95% CI: 0.0–29.5), respectively (Figure 2). Median survival was 2.4 months (95% CI: 0.6–4.1). Although not reaching statistical significance breast cancer patients had a longer median survival (19.0 months) than lung cancer patients (1.4 months; P = 0.577). One-year survival rates were 60.0% and 26.7%, respectively. When the overall survival of patients undergoing further systemic treatment were compared with that of patients referred to palliative care, a statistically significant difference was detected: median survival was 14.2 and 1.4 months, respectively, while 1-year survival rates were 55.6% and 10.0%, respectively (P = 0.011).
FIGURE 2.
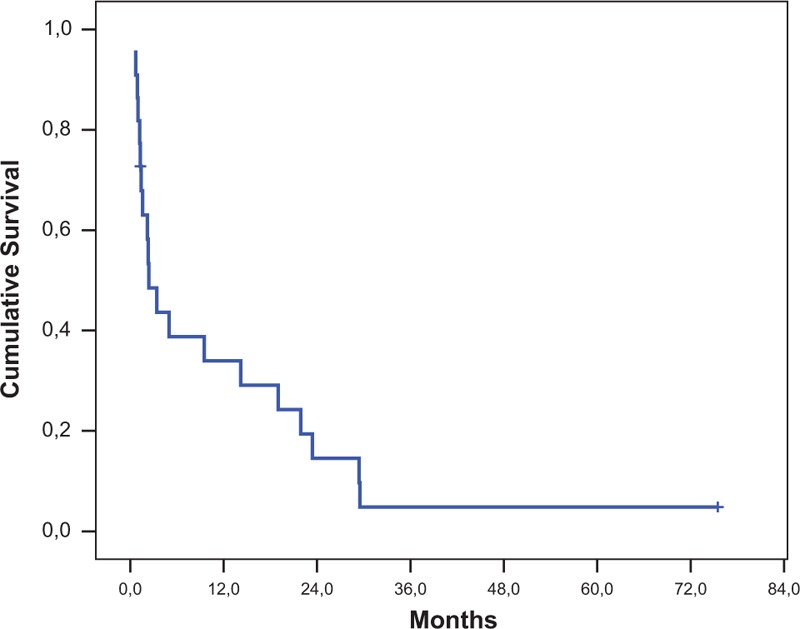
Overall survival.
DISCUSSION
In our series, prompt fluid drainage and pericardial Bleomycin talcage were extraordinarily effective in controlling symptoms and prevented recurrence of effusion in the majority of the patients. We had only 1 treatment failure (5%) and 19% needed a second procedure. Pericardial progression occurred within 6 months in all the cases.
In agreement with available literature, adequate treatment of MPE almost invariably prevents death, and, in suitable patients, affords the administration of systemic treatments. In chemo-sensitive diseases, this can translate into a long life expectancy30,31: we had 7 patients surviving 1 year or more. Moreover, in patients with HER2 positive breast cancer and in lung cancer patients with EGFR mutation positive disease, targeted treatment may potentially obtain even better results.
MPE is a potentially lethal complication of malignancy but, also when resolved, carries a high risk of relapse: in series of pericardicentesis as the sole treatment, the rate of relapse was 33%12 and these data were confirmed in a recent literature review in which was 38%.13 Prevention of relapse should thus be pursued. There are several alternatives to achieve long-term MPE control: extended drainage, the local instillation of anticancer drugs (pericardial talcage), or the creation of a pleuro-pericardial window through a surgical procedure or a minimally invasive maneuver (balloon pericardiotomy).27 All these treatments result in a low incidence of MPE re-accumulation and the best strategy has not been established.
In a randomized trial comparing extended drainage with the addition of Bleomycin intrapericardial administration in patients with lung cancer-related MPE, survival rates with MPE control (effusion-free survival) at 2 months were higher in the Bleomycin arm (29% and 46%, respectively), although this difference did not reach statistical significance.28 In addition to the low number of enrolled patients, another possible reason for lower than expected results is that patients with MPE without the criteria of tamponade were enrolled. The median drainage volume in the Bleomycin arm was 600 mL, with a range from 130 to 1930 mL. In our study, the median fluid volume was 800 mL with a range from 500 to 1350 mL. These data, together with the notion that Bleomycin did not add any toxicity to the extended pericardial drainage, strongly support the addition of Bleomycin administration to the drainage.
Among the various drugs that can be safely injected in the pericardial space, there is not a best option because no comparison is available. Although some authors have made a distinction between sclerosing agents (such as Bleomycin) and antineoplastic agents (such as Cisplatin and Thiotepa), on a clinical ground all these drugs seem to function both as irritating and antineoplastic agents.4 A direct anticancer activity cannot be excluded: in some studies, Cisplatin was found to give better results in lung cancer patients19,32 and a cytological response was found after repeated administrations.33 The worse results obtained in patients with lung cancer-related MPE may support the hypothesis that using a more active drug in this disease (such as Cisplatin) should have obtained better local control.34
In a review examining the published results of the various procedures, extended pericardial drainage was found to result in complete resolution of effusion in only 55% of cases.27 In the same review, the rate of complete resolution of effusion using pericardial sclerosis with either Cisplatin, Thiotepa, Bleomycin, or Mitoxantrone approached 90%, with no evident difference between agents.
Another possible option is surgical decompression through mini-invasive procedures (balloon pericardiotomy, subxiphoid, or thorascopic pericardiostomy) or more extensive surgical treatments (open thoracotomy with pericardial stripping). A pericardial window (which allows drainage of fluid externally or internally such as into the pleural cavity) is often created. Case series have suggested that reaccumulation rates with surgical therapies are low (<15%). However, the complication rate is in the order of 4.5%.35–37 An equal rate of effusion control was reported also in the studies with percutaneous balloon pericardiotomy, although this technique seems to be subject to a higher rate of complications, namely pleural effusion and pneumothorax.27,38–40
We found a dismal prognosis in patients who, due to compromised general conditions, cancer status, or preceding treatments, could not undergo systemic treatments. Among these patients, lung cancer was the predominant primary. These patients had a median survival of 1.5 months, suggesting that MPE relapse should be considered an issue only for patients suitable for systemic treatment.41 We suggest that simple pericardiocentesis may be appropriate for patients with such a short prognosis. This procedure is simple and can be repeated in case of symptomatic relapse. Patients with longer prognoses and suitable for systemic treatments will likely benefit most from sclerosis or surgical decompression; there is no clear evidence currently suggesting that 1 strategy is superior to the other.
Some limitations of our study should be acknowledged: a limited number of patients were enrolled in a long-time span, thus threatening the generalizability of the results. In recent years, treatment of both breast and lung cancer has evolved and better results can be achieved in selected patients with target systemic therapy.
However, the reported data, together with available literature, support the use of Bleomycin talcage as the procedure of choice due to the low invasivity, high success rate, and low toxicity degree.
Acknowledgment
We thank Caroline Oakley for English revision.
Footnotes
Abbreviations: CI = confidence interval, CT = computed tomography, MPE = malignant pericardial effusion.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Klatt EC, Heitz DR. Cardiac metastases. Cancer 1990; 65:1456–1459. [DOI] [PubMed] [Google Scholar]
- 2.Abraham KP, Reddy V, Gattuso P. Neoplasms metastatic to the heart: review of 3314 consecutive autopsies. Am J Cardiovasc Pathol 1990; 3:195–198. [PubMed] [Google Scholar]
- 3.Soler-Soler J, Sagristà-Sauleda J, Permanyer-Miralda G. General cardiology: management of pericardial effusions. Heart 2001; 86:235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lestuzzi C. Neoplastic pericardial disease: old and current strategies for diagnosis and management. World J Cardiol 2010; 2:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shepherd FA. Malignant pericardial effusion. Curr Opin Oncol 1997; 9:170–174. [DOI] [PubMed] [Google Scholar]
- 6.Dosios T, Theakos N, Angouras D, et al. Risk factors affecting the survival of patients with pericardial effusion submitted to subxiphoid pericardiostomy. Chest 2003; 124:242–246. [DOI] [PubMed] [Google Scholar]
- 7.Lamont E, Hoffman PC. Hall JB, Schmidt GA, Wood LDH. Oncologic emergencies. Principles of Critical Care McGraw-Hill, 3rd edNew York, NY:2005. [Google Scholar]
- 8.Laham RJ, Cohen DJ, Kuntz RE, et al. Pericardial effusion in patients with cancer: outcome with contemporary management strategies. Heart 1996; 75:67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang PC, Yang KY, Chao JY, et al. Prognostic role of pericardial fluid cytology in cardiac tamponade associated with non-small cell lung cancer. Chest 2000; 118:744–749. [DOI] [PubMed] [Google Scholar]
- 10.Imazio M, Demichelis B, Parrini I, et al. Relation of acute pericardial disease to malignancy. Am J Cardiol 2005; 95:1393–1394. [DOI] [PubMed] [Google Scholar]
- 11.Vaitkus PT, Herrmann HC, LeWinter MM. Treatment of malignant pericardial effusion. JAMA 1994; 272:59–64. [PubMed] [Google Scholar]
- 12.Apodaca-Cruz A, Villarreal-Garza C, Torres-Avila B, et al. Effectiveness and prognosis of initial pericardiocentesis in the primary management of malignant pericardial effusion. Interact Cardio Vasc Thorac Surg 2010; 11:154–161. [DOI] [PubMed] [Google Scholar]
- 13.Virk SA, Chandrakumar D, Villanueva C, et al. Systematic review of percutaneous interventions for malignant pericardial effusion. Heart 2015; doi:10.1136/heartjnl-2015-307907. [DOI] [PubMed] [Google Scholar]
- 14.Celik S, Lestuzzi C, Cervesato E, et al. Systemic chemotherapy in combination with pericardial window has better outcomes in malignant pericardial effusions. J Thorac Cardiovasc Surg 2014; 148:2288–2293. [DOI] [PubMed] [Google Scholar]
- 15.Maher EA, Shepherd FA, Todd TJR. Pericardial sclerosis as the primary management of malignant pericardial effusion and cardiac tamponade. J Thorac Cardiovasc Surg 1996; 112:637–643. [DOI] [PubMed] [Google Scholar]
- 16.Lashevsky I, Ben Yosef R, Rinkevich D, et al. Intrapericardial minocycline sclerosis for malignant pericardial effusion. Chest 1996; 109:1452–1454. [DOI] [PubMed] [Google Scholar]
- 17.Colleoni M, Martinelli G, Beretta F, et al. Intracavitary chemotherapy with thiotepa in malignant pericardial effusions: an active and well-tolerated regimen. J Clin Oncol 1998; 16:2371–2376. [DOI] [PubMed] [Google Scholar]
- 18.Martinoni A, Cipolla CM, Cardinale D, et al. Long-term results of intrapericardial chemotherapeutic treatment of malignant pericardial effusions with thiotepa. Chest 2004; 126:1412–1416. [DOI] [PubMed] [Google Scholar]
- 19.Maisch B, Ristic AD, Pankuweit S, et al. Neoplastic pericardial effusion. Efficacy and safety of intrapericardial treatment with cisplatin. Eur Heart J 2002; 23:1625–1631. [DOI] [PubMed] [Google Scholar]
- 20.Moriya T, Takiguchi Y, Tabeta H, et al. Controlling malignant pericardial effusion by intrapericardial carboplatin administration in patients with primary non-small-cell lung cancer. Br J Cancer 2000; 83:858–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomkowski WZ, Wisniewska J, Szturmowicz M, et al. Evaluation of intrapericardial cisplatin administration in cases with recurrent malignant pericardial effusion and cardiac tamponade. Supp Care Cancer 2004; 12:53–57. [DOI] [PubMed] [Google Scholar]
- 22.Norum J, Lunde P, Aasebo U, et al. Mitoxantrone in malignant pericardial effusion. J Chemother 1998; 10:399–404. [DOI] [PubMed] [Google Scholar]
- 23.Kaira K, Takise A, Kobayashi G, et al. Management of malignant pericardial effusion with instillation of mitomycin C in non-small cell lung cancer. Jpn J Clin Oncol 2005; 35:57–60. [DOI] [PubMed] [Google Scholar]
- 24.Ostrowski MJ, Halsall GM. Intracavitary bleomycin in the management of malignant effusions: a multicenter study. Cancer Treat Rep 1982; 66:1903–1907. [PubMed] [Google Scholar]
- 25.Yano T, Yokoyama H, Inoue T, et al. A simple technique to manage malignant pericardial effusion with a local instillation of bleomycin in non-small cell carcinoma of the lung. Oncology 1994; 51:507–509. [DOI] [PubMed] [Google Scholar]
- 26.van Belle SJ, Volckaert A, Taeymans Y, et al. Treatment of malignant pericardial tamponade with sclerosis induced by instillation of bleomycin. Int J Cardiol 1987; 16:155–160. [DOI] [PubMed] [Google Scholar]
- 27.Jama GM, Scarci M, Bowden J, et al. Palliative treatment for symptomatic malignant pericardial effusion. Interact Cardiovasc Thorac Surg 2014; 19:1019–1026. [DOI] [PubMed] [Google Scholar]
- 28.Kunitoh H, Tamura T, Shibata T, et al. A randomised trial of intrapericardial bleomycin for malignant pericardial effusion with lung cancer (JCOG9811). Br J Cancer 2009; 100:464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu G, Crump M, Goss PE, et al. Prospective comparison of the sclerosing agents doxycycline and bleomycin for the primary management of malignant pericardial effusion and cardiac tamponade. J Clin Oncol 1996; 14:3141–3147. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda H, Kikawa Y, Nakamoto Y, et al. A patient with recurrent breast cancer showing long-term survival after developing pericardial effusion and cardiac tamponade caused by carcinomatous pericarditis. Breast Care 2013; 8:71–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiong W, Shi C. Malignant pericardial effusion. N Engl J Med 2011; 364:e18. [DOI] [PubMed] [Google Scholar]
- 32.Lestuzzi C, Bearz A, Lafaras C, et al. Neoplastic pericardial disease in lung cancer: impact on outcomes of different treatment strategies. A multicenter study. Lung Cancer 2011; 72:340–347. [DOI] [PubMed] [Google Scholar]
- 33.Lestuzzi C, Lafaras C, Bearz C, et al. Malignant pericardial effusion: sclerotherapy or local chemotherapy? Br J Cancer 2009; 101:734–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lestuzzi C, Viel E, Sorio R, et al. Local chemotherapy for neoplastic pericardial effusion. Am J Cardiol 2000; 86:1292. [DOI] [PubMed] [Google Scholar]
- 35.Niclauss L, Montemurro M, Pretre M. Survival after surgical drainage of malignant pericardial effusion. World J Surg 2015; 39:1767–1772. [DOI] [PubMed] [Google Scholar]
- 36.Moores DW, Allen KB, Faber LP, et al. Subxiphoid pericardial drainage for pericardial tamponade. J Thorac Cardiovasc Surg 1995; 109:546–552. [DOI] [PubMed] [Google Scholar]
- 37.Jeon HW, Cho DG, Park JK, et al. Prognostic factors affecting survival of patients with cancer-related pericardial effusion managed by surgery. World J Surg Oncol 2014; 12:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galli M, Politi A, Pedretti F, et al. Percutaneous balloon pericardiotomy for malignant pericardial tamponade. Chest 1995; 108:1499–1501. [DOI] [PubMed] [Google Scholar]
- 39.Palacios IF, Tuzcu EM, Ziskind AA, et al. Percutaneous balloon pericardial window for patients with malignant pericardial effusion and tamponade. Cathet Cardiovasc Diagn 1999; 22:244–249. [DOI] [PubMed] [Google Scholar]
- 40.Ziskind AA, Pearce AC, Lemmon CC, et al. Percutaneous balloon pericardiotomy for the treatment of cardiac tamponade and large pericardial effusions: description of technique and report of the first 50 cases. J Am Coll Cardiol 1993; 21:1–5. [DOI] [PubMed] [Google Scholar]
- 41.Li BT, Pearson A, Pavlakis N, et al. Malignant cardiac tamponade from non-small cell lung cancer: case series from the era of molecular targeted therapy. J Clin Med 2015; 4:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]


