Figure 8.
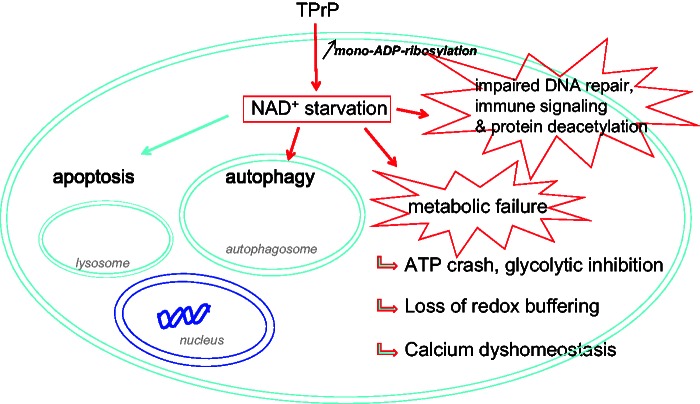
TPrP-induced neuronal death is due to NAD+ deprivation and multiple metabolic failure. Our study suggests the following pathogenic mechanism: TPrP enters neuronal cells and induces excessive protein mono-ADP-ribosylation (an NAD+-consuming reaction) resulting in depletion of intracellular NAD+. NAD+ deprivation leads to metabolic failure: (i) NAD+ is necessary for ATP production; NAD+ is also an essential cofactor in glycolysis; glycolytic inhibition induces mitochondrial failure by blocking the flux of glucose-derived pyruvate; (ii) NAD+ is necessary for cellular redox reactions and the ability of cells to survive oxidative stress via the NAD+/NADH and NADP+/NADPH cycles; and (iii) NAD+ is central for intracellular calcium homeostasis because it is the precursor of calcium-regulating molecules NADP+ and cADPR. NAD+ deprivation also leads to impaired DNA repair responses and protein de-acetylation because of its role as a co-factor in ADP-ribosylation and sirtuin-mediated deacetylation reactions. Besides these effects that directly account for cell death, NAD+ starvation activates autophagy (Billington et al., 2008). Caspase 8- and 9-mediated apoptosis (that we described earlier; Zhou et al., 2012) may be induced directly by NAD+ deprivation. However, we cannot exclude that apoptosis be induced by autophagy, as autophagic induction of apoptosis has been described in some instances (Bhoopathi et al., 2010; Lepine et al., 2011).