Abstract
The aim of the present study was to evaluate 29 whole blood or serum indicators to identify factors able to predict clinical outcome following cytokine-induced killer (CIK) cell therapy combined with chemotherapy in patients with advanced non-small cell lung cancer (NSCLC), and to evaluate the 5-year prognosis of the patients. From March 2008 to October 2013, 42 patients with advanced NSCLC (stages III and IV) were enrolled in the study. These patients were from a single hospital, and had been treated with CIK therapy combined with chemotherapy. Evaluation of the correlation between prognosis and age, gender, tumor stage, surgery resection status, number of CIK therapy cycles, tumor subtype, and the differential whole blood or serum indicators were analyzed by Kaplan-Meier methods and the log-rank test. The prognostic factors were analyzed by Cox proportional models. The median progression-free survival (mPFS) time of patients with high expression levels of albumin [20.0 months; 95% confidence interval (CI): 17.4–22.6 months] was significantly longer than the mPFS for patients with low expression levels of albumin (36.0 months; 95% CI: 24.7–47.3 months) (P=0.034). Other factors demonstrated no significant difference. Following analysis using the Cox proportional hazards regression model, the number of CIK therapy cycles (P=0.041) and the expression level of albumin (P=0.038) were revealed to be independent prognostic factors following the use of CIK cell therapy combined with chemotherapy for patients with advanced NSCLC. The risk of adverse outcomes in patients receiving ≥4 CIK therapy cycles and in patients with increased expression levels of albumin were 0.38 (95% CI: 0.14–1.13) and 0.32 (95% CI: 0.10–1.24)-fold those of patients receiving <4 CIK therapy cycles and with decreased expression levels of albumin, respectively. The serum albumin concentration may therefore be a predictor of the 5-year survival rate of patients with advanced NSCLC treated with CIK cell therapy combined with chemotherapy; patients with high expression levels of albumin may have a better prognosis in comparison with patients with low expression levels of albumin.
Keywords: non-small cell lung cancer, cytokine-induced killer, chemotherapy, prognosis, albumin
Introduction
Lung cancer is one of the most common cancers worldwide. In the USA, an estimated 226,160 new cases were diagnosed in 2012, accounting for 14% of cancer diagnoses. In addition, lung cancer accounts for more mortalities than any other type of cancer; an estimated 160,340 mortalities were reported in 2012, accounting for 28% of all cancer-related mortalities (1). In China, lung cancer replaced liver cancer as the leading cause of mortality in patients with malignant tumors in 2008, and the mortality rate has increased by 464.84% in the past three decades (2). Lung cancer has two major forms, namely small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC); the latter of these accounts for 85–90% of lung cancer cases (3). As early detection methods are lacking, the majority of NSCLC patients are diagnosed at an advanced stage of disease (4). At present, the most common treatment approaches for advanced NSCLC in clinical practice include surgery, chemotherapy and radiation therapy; however, the prognosis of surgery is poor due to the high incidence of recurrence. The effects of chemotherapy and radiation therapy are also limited, associated with drug resistance and adverse reactions (5). Therefore, novel and effective therapeutic strategies are urgently required for advanced NSCLC in clinical therapy.
With the rapid development of cellular and molecular immunology, the pathogenesis of cancer has been closely linked to the host immune system (6). A number of researchers and doctors have recognized immunological therapy as a promising therapeutic approach in the treatment of cancer when following surgery, chemotherapy and radiation therapy (7,8). One type of immunological therapy uses cytokine-induced killer (CIK) cells, which are peripheral blood mononuclear cells (PBMCs) that have been stimulated by various cytokines [interferon (IFN)-γ, anti-cluster of differentiation (CD)3 antibodies, interleukin (IL)-1 and/or IL-2]. This allows for the proliferation of effector cells, which include non-specific T cells and natural killer (NK) cells, with lymphokine-activated killer (LAK) activity. These proliferative CIK cells simultaneously express the T-cell marker CD3 and the NK cell marker CD56, which provide cytotoxic effects in order to kill tumor cells. The CIK cells combine the marked anticancer activity of T cells with the non-major histocompatibility complex (MHC)-restricted anticancer activity of NK cells (9). Compared with conventional therapeutic approaches, CIK therapy has several advantages, such as increased anticancer activity, a broader anticancer spectrum, the ability to prevent tumor recurrence, and fewer adverse reactions (10). CIK cells have been evaluated as an adoptive immunotherapy by a number of clinical trials investigating their clinical applicability in the treatment of various kinds of cancers, including liver cancer (11), lung cancer (12), melanoma (13), gastrointestinal cancer (14) and renal cell carcinoma (15,16).
The efficacy and safety of chemotherapy and CIK immunotherapy co-treatment and chemotherapy alone for advanced NSCLC have been evaluated in previous studies; however, these have mainly focused on short-time overall survival (OS; <2 years), progression free survival (PFS) and response rate (12,17–19). In addition, few studies on factors predictive of clinical outcome in immune therapy have been performed (20–23). Therefore, an effective prognostic factor is urgently required to improve the effectiveness of CIK therapy in advanced NSCLC patients. The aim of the present study was to evaluate 29 whole blood or serum indicators to identify factors that are predictive of clinical outcome following CIK cell therapy combined with chemotherapy, and to evaluate the 5-year prognosis of patients with advanced NSCLC.
Materials and methods
Patient characteristics
The present study was approved by the Ethics Committee of Chinese PLA General Hospital (Beijing, China). Written informed consent was obtained from all patients. All the procedures in this study were conducted in accordance with the Declaration of Helsinki. From March 2008 to October 2013, 42 patients with advanced NSCLC (stages III and IV) were enrolled at the Biotherapeutic Department of the Chinese PLA General Hospital. All of the patients with NSCLC were administered 4–6 cycles of cisplatin- or carboplatin-based chemotherapy regimens, including gemcitabine, pemetrexed or docetaxel. All patients were treated with CIK cell therapy 7–10 days following chemotherapy. CIK cell treatment was administered at 1-month intervals. All 42 patients were eligible for CIK maintenance treatment until they no longer agreed to continue maintenance treatment or until disease progression occurred. For each therapy cycle, patients were given an infusion of 2–10×109 CIK cells. Data concerning age, gender, tumor stage, surgery resection status, CIK therapy cycle and tumor subtype were also collected. Patient characteristics are shown in Table I.
Table I.
Characteristics of the 42 patients enrolled in the study.
| Characteristic | Number |
|---|---|
| Age | |
| ≥60 years | 21 |
| <60 years | 21 |
| Gender | |
| Male | 25 |
| Female | 17 |
| Stage | |
| III | 11 |
| IV | 31 |
| Surgery resection | |
| Yes | 8 |
| No | 34 |
| Therapy cycles | |
| ≥4 | 22 |
| <4 | 20 |
| Type | |
| Squamous | 30 |
| Adenocarcinoma | 6 |
| Others | 6 |
Preparation of CIK cells
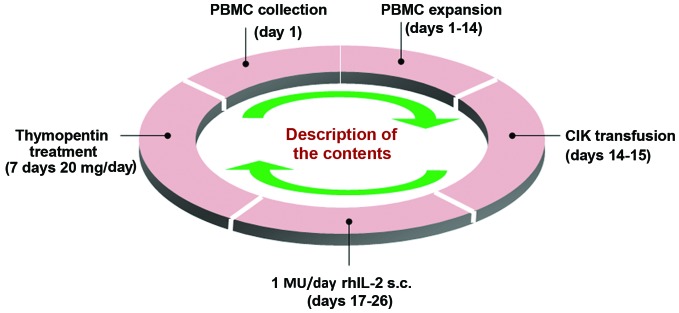
All procedures used in CIK cell preparation were performed within a sterile environment. Expansion and culture of the CIK cells was performed in accordance with a previous method (24). Peripheral blood (50 ml) was collected, and PBMCs were extracted by Ficoll-Paque density-gradient centrifugation (GE Healthcare Life Sciences, Chalfont, UK). PBMCs were then washed three times in PBS, and suspended in GT-T551 medium (2×106 cells/ml; Takara Bio, Inc., Otsu, Japan), seeded into a 75-cm2 culture flask, and then placed into a 5% CO2 incubator at 37°C. After 6 h, the liquid was transferred into a new 75-cm2 culture flask, which had been pretreated with phosphate-buffered saline containing 5 µg/ml mouse anti-CD3 monoclonal antibody (cat. no. T210; Takara Bio, Inc.) at 4°C overnight. This medium was then supplemented with 0.6% serum from the patient. On day 0, 1,000 U/ml recombinant human interleukin-2 (rhIL-2; Peprotech, Inc. Rocky Hill, NJ, USA) and 10,000 U/ml recombinant IFN-γ (Peprotech Inc.) were added to the culture medium. The cells were cultured in a humidified 5% CO2 incubator at 37°C. Fresh GT-T551 medium with 1,000 U/ml rhIL-2 was added every 3 days. CIK cells were harvested on day 14. The collection criteria were as follows: i) The proportions of CD3+, CD3+CD8+ and CD3+CD56+ cells were >95, >80 and >20%, respectively, as determined by flow cytometry; ii) cell viability, which was measured by Trypan blue staining, was >95%; iii) contamination, such as bacteria, fungi, endotoxins and Mycoplasma, could not be detected (as assessed by researchers from the Department of Microbiology, Chinese PLA General Hospital, Beijing, China); iv) the total number of cells was 2–10×109. The CIK cell therapy cycle is illustrated in Fig. 1.
Figure 1.
Flowchart of the CIK cell therapy. PBMC, peripheral blood mononuclear cell; CIK, cytokine-induced killer; rhIL-2, recombinant human interleukin 2; s.c., subcutaneously.
Flow cytometric analysis of phenotype
The CIK cells were resuspended in 100 µl of PBS containing 15 µl of the following monoclonal mouse antibodies: anti-CD4-fluorescein isothiocyanate (FITC), anti-CD8-phycoerythrin (PE), anti-CD3-chlorophyll protein complex (PerCP), included within a Tritest kit (cat. no. 340298), in addition to 5 µl anti-CD56-allophycocyanin (APC; cat. no. 555518) in the dark for 30 min at 4°C, and then washed twice in PBS. The antibodies and isotype control antibodies were purchased from BD Biosciences (San Jose, CA, USA). These were used to stain cell surface markers in order to identify the CIK phenotype. Data acquisition was performed using a FACSCalibur flow cytometer (BD Biosciences).
Detection of 29 whole blood or serum indicators
A total of 8 whole blood indicators that were tested, namely hemoglobin, white blood cell (WBC) count, red blood cell (RBC) count, platelet count, lymphocytes, monocytes, basophils and eosinophils. These were detected using an XE-2100 Automated Hematology System kit (Sysmex Corporation). A total of 21 serum indicators were tested, which were: Lactate dehydrogenase (LDH), alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total protein (TP), albumin (ALB), total bilirubin (TB), direct bilirubin (DB), total bile acid (TBA), γ-glutamyl aminotransferase (γGGT), urea (UR), creatinine (CR), uric acid (UA), carbon dioxide (CO2), neuron-specific enolase (NSE), carcino-embryonic antigen (CEA), α-fetoprotein (AFP), carbohydrate antigen (CA)125, CA724, CA153 and CA199. Detection kits for LDH, ALP, ALT, AST, TP, ALB, γGGT, UR, CR, UA, CO2, NSE, CEA, AFP, CA125, CA724, CA153 and CA199 were provided by Roche Diagnostics (Basel, Switzerland). DB and TB detection kits were provided by Hitachi Chemical Diagnostics (Mountain View, CA, USA). TBA detection kits were provided by Strong Biotechnologies (Beijing, China). LDH, ALP, ALT, AST, TP, ALB, γGGT, UR, CR, UA, CO2, DB, TB and TBA were detected using a Hitachi 7600 automatic biochemical analyzer (Hitachi, Tokyo, Japan). NSE, CEA, AFP, CA125, CA724, CA153, and CA199 were detected using a Roche E170EE automated immunoassay analyzer (Roche Diagnostics).
Prognosis evaluation
The median progress free survival (mPFS) was used to evaluate the prognosis of the patients with advanced NSCLC receiving CIK cell therapy combined with chemotherapy. Patients without a known date of mortality were reported as indicated at the time of the last follow-up.
Statistical analysis
All statistical analysis was performed using SPSS software (version 13 for Windows; SPSS, Inc., Chicago, IL, USA) and GraphPad Prism (version 6 for Windows; GraphPad Software, Inc., La Jolla, CA, USA). All data are reported as median (25th percentile, 75th percentile). The indicators were compared between pre- and post-therapy by paired t-test, and the indicators between different CIK cell therapy cycles were also compared by paired t-test. The survival curves were calculated by the Kaplan-Meier method, and differences between survival curves were compared by the log-rank test. Log-rank tests were also used to conduct the single factor analysis for age, gender, tumor stage, surgery resection status, CIK therapy cycle, tumor subtype information, and differential whole blood or serum indicators. Multivariate analysis was performed using the Cox's proportional hazards regression model to evaluate the significance of prognostic factors. P-values <0.05 were considered to indicate a significant difference.
Results
Comparison of peripheral lymphocyte subsets
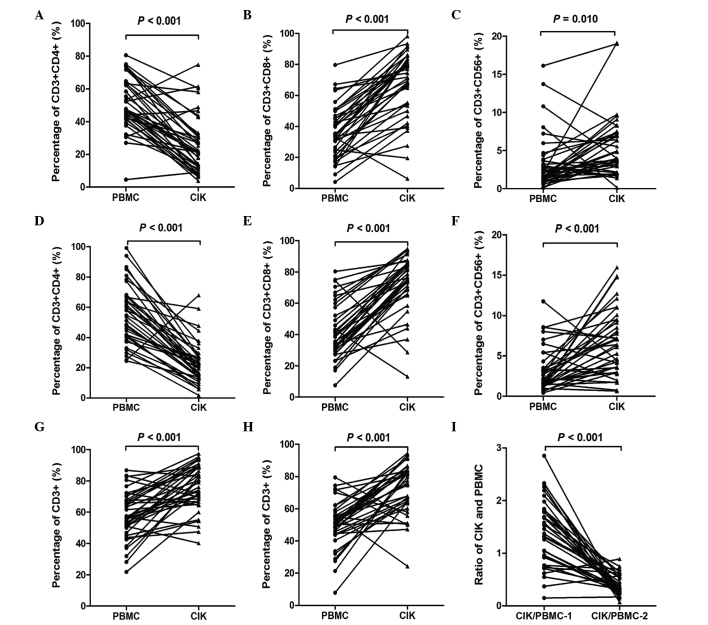
The percentages of CD3+, CD3+CD4+, CD3+CD8+, CD3+CD56+ and CD8+CD56+peripheral lymphocyte subsets, and the CIK/PBMC ratio in the first and second cycles of therapy were compared with those in PBMCs, as reported in Fig. 2. The percentage of CD3+CD4+ lymphocytes in the CIK group were significantly reduced when compared with the PBMC group in the first cycle (P<0.001) and second cycle (P<0.001) of therapy (Fig. 2A and D). The percentage of CD3+CD8+ lymphocytes in the CIK group revealed a significant increase when compared with the PMBC group in the first cycle (P<0.001) and second cycle (P<0.001) of therapy (Fig. 2B and E). The percentage of CD3+CD56+ lymphocytes in the CIK group was also significantly increased when compared with that in the PBMC group in the first (P=0.001) and second cycles (P<0.001) of therapy (Fig. 2C and F). Furthermore, the percentage of total CD3+ lymphocytes in the CIK group was significantly increased when compared with that in the PBMC group in the first cycle (P<0.001) and second cycle (P<0.001) of therapy (Fig. 2G and H). The CIK/PBMC ratio in the first and second cycles of therapy was also compared. The CIK/PBMC ratio in the second cycle was significantly reduced when compared with that in the first cycle of therapy (P<0.001). However, no significant difference was observed between the percentage of total CD8+CD56+ lymphocytes in the CIK group and the PBMC group following the first and second cycles of therapy (both P>0.05; data not shown).
Figure 2.
Comparison of the percentages of peripheral lymphocyte subsets and the ratio of CIK/PBMC in the first cycle and second cycle of therapy. (A) CD3+CD4+ lymphocytes, (B) CD3+CD8+ lymphocytes and (C) CD3+CD56+ lymphocytes in the first cycle; (D) CD3+CD4+ lymphocytes; (E) CD3+CD8+ lymphocytes and (F) CD3+CD56+ lymphocytes in the second cycle; CD3+ lymphocytes in (G) the first cycle and (H) the second cycle; (I) The ratio of CIK and PBMC in the first cycle (CIK/PBMC-1) and second cycle (CIK/PBMC-1) CIK, cytokine-induced killer; PBMC, peripheral blood mononuclear cell; CD, cluster of differentiation.
Comparison of whole blood routine and serum biomarker tests
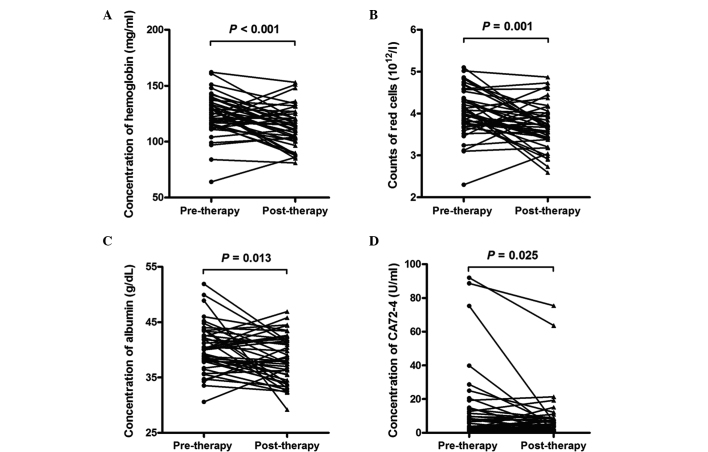
In addition to conducting an analysis of immune indicators, 29 whole blood and serum biomarker indicators were analyzed, the results of which are shown in Table II. The hemoglobin (P<0.001) and RBC counts (P=0.001) of the whole blood analyses were significantly reduced in the advanced lung cancer patients post-therapy when compared with those pre-therapy (Fig. 3A and B). The levels of hemoglobin in the pre-therapy group were 125.00 (115.75, 135.50) mg/ml, and in the post-therapy group were 112.00 (102.50, 122.00) mg/ml. The RBC count in the pre-therapy group was 4.03 (3.74, 4.41) ×1012/l, and in the post-therapy group was 3.67 (3.40, 4.01) ×1012/l. No significant difference in the other indicators in the whole blood analyses (WBC count, platelet count, lymphocytes, monocytes, basophils and eosinophils) were observed between pre-therapy and post-therapy in the patients with advanced lung cancer. In the serum biomarker test, the ALB (P=0.013) and CA724 (P=0.025) levels revealed a significant reduction in the patients with advanced lung cancer post-therapy compared with those pre-therapy (Fig. 3C and D). The levels of ALB in the pre-therapy group were 40.10 (37.88, 43.43) g/dl, and in the post-therapy were 37.95 (34.38, 41.71) g/dl. The levels of CA724 in the pre-therapy group were 6.10 (1.91, 14.20) U/ml, and in the post-therapy group were 4.92 (2.05, 8.47) U/ml. The other indicators in the serum biomarker test (including LDH, ALP, ALT, AST, TP, TB, DB, TBA, γGGT, UR, CR, UA, CO2, NSE, CEA, AFP, CA153, CA199, and CA125) revealed no observable significant difference between pre-therapy and post-therapy in the advanced lung cancer patients.
Table II.
Comparison of 29 whole blood routine and serum biomarker indicators between pre-and post-therapy in 42 patients with advanced lung cancer.
| Indicator | Pre-therapy | Post-therapy | P-value |
|---|---|---|---|
| Hemoglobin, mg/ml | 125.00 (115.75, 135.50) | 112.00 (102.50,122.00) | <0.001a |
| WBC count, ×109/l | 5.36 (4.66, 7.04) | 5.17 (4.28, 6.98) | 0.795 |
| RBC count, ×1012/l | 4.03 (3.74, 4.41) | 3.67 (3.40, 4.01) | 0.001a |
| Platelet count, ×109/l | 246 (184.50, 297.50) | 255.5 (185.50, 310.00) | 0.163 |
| Lymphocytes, % | 0.27 (0.21, 0.31) | 0.313 (0.210, 0.37) | 0.400 |
| Monocytes, % | 0.72 (0.61, 0.90) | 0.076 (0.063, 0.093) | 0.575 |
| Basophils,% | 0.003 (0.002, 0.006) | 0.004 (0.002, 0.007) | 0.918 |
| Eosinophils, % | 0.014 (0.09, 0.028) | 0.02 (0.010, 0.037) | 0.058 |
| LDH, U/l | 173.6 (138.6, 244.9) | 182.075 (138.75, 246.48) | 0.772 |
| ALP, U/l | 78.25 (61.20, 99.15) | 71.3 (60.50, 96.48) | 0.992 |
| ALT, U/l | 17.05 (13.88, 26.60) | 17.7 (14.04, 26.60) | 0.341 |
| AST, U/l | 19.35 (15.30, 23.55) | 18.6 (16.05, 26.80) | 0.971 |
| TP, g/l | 64.70 (60.60, 72.20) | 64.6 (62.55, 68.00) | 0.147 |
| ALB, g/dl | 40.10 (37.88, 43.43) | 37.95 (34.38, 41.71) | 0.013a |
| TB, µmol/l | 9.85 (7.83, 11.95) | 8.65 (6.38, 11.20) | 0.190 |
| DB, µmol/l | 2.85 (2.08, 4.23) | 2.5 (1.79, 4.35) | 0.902 |
| TBA, µmol/l | 3.75 (2.95, 6.78) | 3.2 (2.55, 4.25) | 0.119 |
| γGGT, U/l | 33.33 (21.50, 56.98) | 33.25 (23.15, 61.33) | 0.668 |
| UR, mmol/l | 4.78 (3.79, 5.70) | 4.26 (3.40, 5.52) | 0.470 |
| CR, µmol/l | 66 (56.20, 73.10) | 61.73 (52.04, 79.96) | 0.425 |
| UA, µmol/l | 286.6 (248.53, 324.65) | 274.45 (230.23, 353.80) | 0.830 |
| CO2, mmol/l | 27 (25.68, 28.73) | 27.33 (26.05, 28.48) | 0.389 |
| NSE, ng/ml | 11.55 (9.75, 16.69) | 12.55 (10.07, 19.13) | 0.338 |
| CEA, ng/ml | 5.24 (1.81, 19.04) | 6.25 (2.91, 56.10) | 0.489 |
| AFP, U/l | 2.84 (2.13, 3.92) | 3.02 (2.37, 3.96) | 0.552 |
| CA724, U/ml | 6.1 (1.91, 14.20) | 4.92 (2.05, 8.47) | 0.025a |
| CA153, U/ml | 20.01 (13.62, 32.60) | 27.43 (16.57, 51.93) | 0.237 |
| CA199, U/ml | 12.04 (6.14, 20.03) | 12.8 (8.34, 38.64) | 0.134 |
| CA125, U/ml | 53.38 (19.75, 110.40) | 30.34 (17.69, 124.60) | 0.462 |
Values are presented as median (25th percentile, 75th percentile).
Significant difference between pre- and post-therapy. WBC, white blood cell; RBC, red blood cell; LDH, lactate dehydrogenase; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TP, total protein; ALB, albumin; TB, total bilirubin; DB, direct bilirubin; TBA, total bile acid; γGGT, γglutamyl aminotransferase; UR, urea; CR, creatinine; UA, uric acid; CO2, carbon dioxide; NSE, neuron-specific enolase; CEA, carcino-embryonic antigen; CA, carbohydrate antigen.
Figure 3.
Differential whole blood or serum indicators pre-therapy and post-therapy in patients with advanced lung cancer. (A) Hemoglobin concentration, (B) red blood cell count, (C) albumin concentration and (D) carbohydrate antigen 72–4.
Differential indicators in continuous therapy cycles
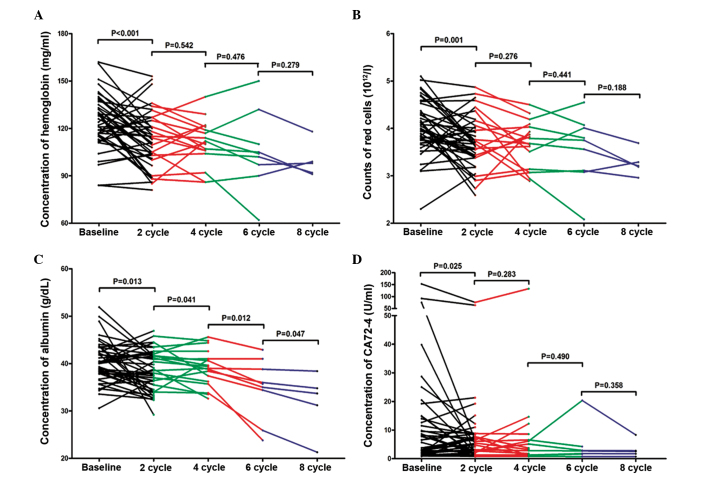
Following analysis of the differential indicators between pre- and post-therapy in the advanced lung cancer patients, the differential indicators hemoglobin, RBC, ALB, and CA724 were compared between different numbers of continuous therapy cycles. No significant difference in concentration of hemoglobin was observed between 2 vs 4 cycles (P=0.542), 4 vs. 6 cycles (P=0.476) or 6 vs. 8 cycles (P=0.279; Fig. 4A). No observably significant difference in RBC counts was detected between 2 vs. 4 cycles (P=0.276), 4 vs. 6 cycles (P=0.441) and 6 vs. 8 cycles (P=0.188; Fig. 4B). However, the data report in Fig. 4C reveal that the concentration of albumin was significantly reduced as the number of therapy cycles increased; 2 vs. 4 cycles (P=0.041), 4 vs. 6 cycles (P=0.012), 6 vs. 8 cycles (P=0.047). The concentration of albumin at 4, 6 and 8 cycles of therapy was 36.64 (36.08, 41.40), 35.70 (30.15, 39.90) and 33.70 (26.25, 36.60) g/dl, respectively. No significant difference was observed in concentration of CA724 between 2 vs. 4 cycles (P=0.283), 4 vs. 6 cycles (P=0.490) or 6 vs. 8 cycles (P=0.358; Fig. 4D).
Figure 4.
Differential whole blood or serum indicators in continuous therapy cycles. (A) Hemoglobin, (B) red blood cell count, (C) albumin and (D) carbohydrate antigen A72–4.
Univariate and multivariate survival analysis
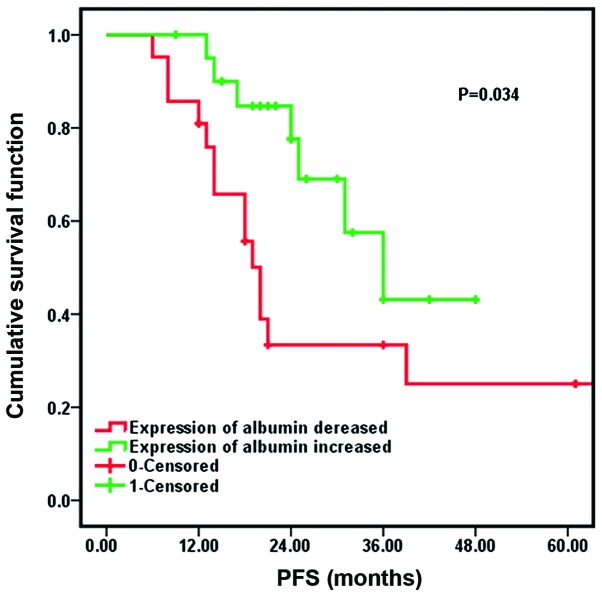
The results of log-rank test analysis, as shown in Table III, indicated that age, gender, tumor stage, surgery resection status, the number of CIK therapy cycles and tumor subtype were not significant predictors of PFS (P>0.05). The mPFS time of the patients with high expression levels of albumin [20.0 months, 95% confidence interval (CI): 17.4–22.6] was longer than the mPFS for patients with low expression levels of albumin (36.0 months, 95% CI: 24.7–47.3), as reported in Fig. 5. Following Cox regression analysis, it was identified that the expression of albumin had a significant effect on the prognosis of patients following CIK cell therapy combined with chemotherapy (P=0.034).
Table III.
Univariate analysis of factors for prognosis analysis of patients with advanced lung cancer treated with CIK cell therapy combined with chemotherapy.
| Kaplan-Meier single factor analysis | ||||
|---|---|---|---|---|
| Correlative factor | No. | mPFS (months) | 95% CI | P-value |
| Age | 0.219 | |||
| ≥60 years | 21 | 24.0 | 17.0–36.0 | |
| <60 years | 21 | 39.0 | 18.0–41.0 | |
| Gender | 0.350 | |||
| Male | 25 | 21.0 | 6.8–35.2 | |
| Female | 17 | 36.0 | 19.2–52.7 | |
| Stage | 0.287 | |||
| III | 11 | 48.0 | 32.3–63.7 | |
| IV | 31 | 33.4 | 24.6–43.1 | |
| Surgical resection | 0.555 | |||
| Yes | 8 | 31.0 | 14.7–47.3 | |
| No | 34 | 25.0 | 6.3–43.7 | |
| Therapy cycles | 0.058 | |||
| ≥4 | 22 | 39.0 | 22.0–55.9 | |
| <4 | 20 | 20.0 | 14.1–25.9 | |
| Type | 0.148 | |||
| Squamous | 30 | 37.7 | 28.0–47.5 | |
| Adenocarcinoma | 6 | 43.0 | 17.7–68.3 | |
| Others | 6 | 22.2 | 7.8–36.5 | |
| Albumin | 0.034a | |||
| Increased | 19 | 20.0 | 17.4–22.6 | |
| Decreased | 23 | 36.0 | 15.8–46.2 | |
CIK, cytokine-induced killer; mPFS, median progression free survival; CI, confidence interval.
Significant difference.
Figure 5.
Comparison of median progression-free survival (PFS) in patients with increased expression of albumin and decreased expression of albumin.
A Cox proportional hazards regression model was then used to conduct a multivariate analysis of age, gender, tumor stage, surgery resection status, CIK therapy cycles, tumor subtype, and the expression of albumin, and the results are reported in Table IV. The number of CIK therapy cycles (P=0.041) and the concentration of albumin (P=0.038) were demonstrated to be independent indicators in the prognosis of CIK cell therapy combined with chemotherapy for patients with advanced NSCLC. The risk of an adverse clinical outcome in patients receiving ≥4 CIK therapy cycles patients was 0.38 (95% CI: 0.14–1.13)-fold that of the patients receiving <4 CIK therapy cycles. The risk of an adverse clinical outcome in patients with increased expression levels of albumin was 0.32 (95% CI: 0.10–1.24)-fold that of patients with lower expression levels of albumin; patients with high expression levels of albumin and receiving more CIK therapy cycles may therefore have a better prognosis compared with patients with low expression levels of albumin and receiving fewer treatment cycles. Other factors, including age, gender, tumor stage, surgery resection status and tumor subtype, did not appear to be independent prognostic factors (P>0.05).
Table IV.
Multivariate analysis (Cox model) of factors for prognosis analysis of patients with advanced lung cancer treated with CIK cell therapy combined with chemotherapy.
| Cox multiple factor analysis | |||
|---|---|---|---|
| Correlative factor | P-value | Exp(B) | 95% CI |
| Age | 0.109 | 2.31 | 0.83–6.41 |
| ≥60 years | |||
| <60 years | |||
| Gender | 0.992 | 1.01 | 0.32–3.13 |
| Male | |||
| Female | |||
| Stage | 0.236 | 2.47 | 0.55–11.05 |
| III | |||
| IV | |||
| Surgical resection | 0.363 | 0.47 | 0.10–2.37 |
| Yes | |||
| No | |||
| Therapy cycles | 0.041a | 0.38 | 0.14–1.13 |
| ≥4 | |||
| <4 | |||
| Type | 0.166 | 1.54 | 0.84–2.82 |
| Squamous | |||
| Adenocarcinoma | |||
| Others | |||
| Albumin | 0.038a | 0.32 | 0.10–1.24 |
| Increased | |||
| Decreased | |||
CIK, cytokine-induced killer; Exp(B), hazard ratio; CI, confidence interval.
Discussion
The primary treatment approaches for cancers in clinical practice are surgical resection, chemotherapy and radiotherapy; however, efficacy can be poor due to high rates of recurrence and a poor prognosis. With the development of immunology and understanding of the etiology of cancer development, immunological therapy has emerged as a promising treatment approach for cancers. Previous studies have demonstrated that the adaptive and innate cellular immunity are important for antitumor effects (25–27). Antitumor cellular immune activity can be markedly enhanced by increased lymphocyte number (28,29). Immunological therapy is conducted using autologous T cells or NK cells, which are isolated from the cancer patients, activated and expanded in vitro; the expanded T cells or NK cells are then re-infused in vivo into the cancer patients. These expanded cells simultaneously express the T-cell marker CD3 and the NK cell marker CD56, which provides the cells with cytotoxic activity, enabling them to kill tumor cells (30). Immunotherapy is a promising therapy in the advancement of cancer treatment; however, the efficacy of CIK therapy in the treatment of NSCLC remains controversial (17,31). The main reason for this controversy is that there is no systematic, multi-center, convincing clinical evidence to support the use of this therapy in NSCLC. Although numerous studies have been performed to investigate CIK therapy of NSCLC, the majority of the studies have focused mainly on short-term survival and the adverse reactions (32–34). Studies on the factors associated with clinical outcome of immune therapy are lacking. In the present study, by evaluating the predictive value of 29 whole blood or serum indicators, the aim was to identify a predictive factor for the 5-year survival prognosis of CIK cell therapy combined with chemotherapy in patients with advanced NSCLC. The therapeutic effects of immunotherapy are considered to be mainly dependent upon boosting the immune system to exhibit anticancer activity.
In the present study, the percentage of CD3+CD56+ lymphocytes in the CIK group significantly increased when compared with that in the PBMC group in the first and second cycles. This is consistent with the findings of previous studies (35,36), and demonstrated the expansion of CD3+CD56+ T-cells in populations of PBMCs in the present study. Albumin is known as a negative acute-phase protein in inflammation (37). The reduction in albumin concentration may be used as a biomarker of inflammation (38). In addition, malnutrition and cachexia in cancer patients are current problems (39), associated with the host response to tumor and anticancer therapies, and ultimately result in poor survival (40). Serum albumin is a useful indicator for estimating visceral protein function; in the advanced stage of cancer, malnutrition and inflammation suppress albumin synthesis (41). Lower levels of serum albumin have been associated with poor survival across numerous studies (42–45). In the present study, it was revealed that the patients with high expression levels of albumin had a relatively better prognosis when compared with patients with low expression levels of albumin, which is consistent with the previous studies. Serum albumin levels have also been associated with autoimmune symptoms, and the appearance of autoimmune symptoms may be a predictive indicator of better survival and prognosis following cancer treatment (46). In patients with thyroid cancer, serum thyroid auto-antibodies have been identified to be a positive prognostic indicator (47). For melanoma patients treated with IFN-α2b, autoimmune symptoms were demonstrated to predict better overall survival rates (48,49). These studies indicate that autoimmune symptoms may positively correlate with the survival time of patients. Immune cell infiltration by T helper 1 cells and cytotoxic T cells may also be a promising prognostic indicator (50). MHC class I chain-related gene A (MICA) protein was demonstrated to be a potential indicator of the clinical outcome in patients with advanced NSCLC (20,51). In addition, immunology score and tumor-associated antigens may predict the outcome of immunotherapy (50,52). Until now, the association between autoimmune symptoms and the prognosis in patients with advanced NSCLC was unknown. The present study revealed that albumin concentration may be an indicator of the clinical outcome in patients with advanced NSCLC.
There are certain limitations to the present study. First, the sample size was relatively small (the number of patients was only 42) and following 2, 4, 6 and 8 cycles, the numbers of matched patients were even fewer. This may create a bias in the results, thus a greater number of patients are required in future studies. Second, the present study was only performed in a single center, and the results may be affected by the patients enrolled, ages, gender, and so on. Multi-center validation should be performed to provide more representative results. Third, the present study is prospective; a retrospective study should be performed to validate the results in this study. Fourth, due to inconsistency of therapeutic standards between the present and previous studies, standardization of the therapy process is necessary to ensure comparability between studies. Lastly, any other factors that may cause high expression of albumin should be investigated. Further studies are required to evaluate the effect of other factors on the prognosis in advanced NSCLC patients.
In conclusion, continuous evaluation of the expression of albumin in serum in patients with advanced NSCLC may help to predict the 5-year survival prognosis of the patients. Patients with high expression levels of albumin may have a relatively better prognosis compared with patients with low expression levels of albumin. However, the expression of albumin should be detected continuously in order to eliminate any other factors that may cause abnormal increases in albumin levels. The present study provides a potential prognostic indicator following CIK cell therapy combined with chemotherapy in the treatment of advanced NSCLC, and builds a theoretical basis for the personalized treatment for patients with advanced NSCLC.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (nos. 31270820, 81230061), National Basic Science and Development Program of China (no. 2012CB518103).
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.She J, Yang P, Hong Q, Bai C. Lung cancer in China: Challenges and interventions. Chest. 2013;143:1117–1126. doi: 10.1378/chest.11-2948. [DOI] [PubMed] [Google Scholar]
- 3.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: Epidemiology, risk factors, treatment and survivorship. Mayo Clin Proc. 2008;83:584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiro SG, Tanner NT, Silvestri GA, Janes SM, Lim E, Vansteenkiste JF, Pirker R. Lung cancer: Progress in diagnosis, staging and therapy. Respirology. 2010;15:44–50. doi: 10.1111/j.1440-1843.2009.01674.x. [DOI] [PubMed] [Google Scholar]
- 5.Pallis AG, Serfass L, Dziadziusko R, van Meerbeeck JP, Fennell D, Lacombe D, Welch J, Gridelli C. Targeted therapies in the treatment of advanced/metastatic NSCLC. Eur J Cancer. 2009;45:2473–2487. doi: 10.1016/j.ejca.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: Impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 7.Fischbach MA, Bluestone JA, Lim WA. Cell-based therapeutics: The next pillar of medicine. Sci Transl Med. 2013;5:179ps7. doi: 10.1126/scitranslmed.3005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sangiolo D. Cytokine induced killer cells as promising immunotherapy for solid tumors. J Cancer. 2011;2:363–368. doi: 10.7150/jca.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu PH, Negrin RS. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol. 1994;153:1687–1696. [PubMed] [Google Scholar]
- 10.Jiang J, Wu C, Lu B. Cytokine-induced killer cells promote antitumor immunity. J Transl Med. 2013;11:83. doi: 10.1186/1479-5876-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang FS, Liu MX, Zhang B, Shi M, Lei ZY, Sun WB, Du QY, Chen JM. Antitumor activities of human autologous cytokine-induced killer (CIK) cells against hepatocellular carcinoma cells in vitro and in vivo. World J Gastroenterol. 2002;8:464–468. doi: 10.3748/wjg.v8.i3.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L, Ren B, Li H, Yu J, Cao S, Hao X, Ren X. Enhanced antitumor effects of DC-activated CIKs to chemotherapy treatment in a single cohort of advanced non-small-cell lung cancer patients. Cancer Immunol Immunother. 2013;62:65–73. doi: 10.1007/s00262-012-1311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gammaitoni L, Giraudo L, Leuci V, Todorovic M, Mesiano G, Picciotto F, Pisacane A, Zaccagna A, Volpe MG, Gallo S, et al. Effective activity of cytokine-induced killer cells against autologous metastatic melanoma including cells with stemness features. Clin Cancer Res. 2013;19:4347–4358. doi: 10.1158/1078-0432.CCR-13-0061. [DOI] [PubMed] [Google Scholar]
- 14.Jakel CE, Vogt A, Gonzalez-Carmona MA, Schmidt-Wolf IG. Clinical studies applying cytokine-induced killer cells for the treatment of gastrointestinal tumors. J Immunol Res. 2014;2014:897214. doi: 10.1155/2014/897214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Wang J, Wang Y, Lu XC, Fan H, Liu Y, Zhang Y, Feng KC, Zhang WY, Chen MX, et al. Autologous CIK cell immunotherapy in patients with renal cell carcinoma after radical nephrectomy. Clin Dev Immunol. 2013;2013:195691. doi: 10.1155/2013/195691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JS, Chung IS, Lim SH, Park Y, Park MJ, Kim JY, Kim YG, Hong JT, Kim Y, Han SB. Preclinical and clinical studies on cytokine-induced killer cells for the treatment of renal cell carcinoma. Arch Pharm Res. 2014;37:559–566. doi: 10.1007/s12272-014-0381-x. [DOI] [PubMed] [Google Scholar]
- 17.Wu C, Jiang J, Shi L, Xu N. Prospective study of chemotherapy in combination with cytokine-induced killer cells in patients suffering from advanced non-small cell lung cancer. Anticancer Res. 2008;28:3997–4002. [PubMed] [Google Scholar]
- 18.Shi SB, Ma TH, Li CH, Tang XY. Effect of maintenance therapy with dendritic cells: Cytokine-induced killer cells in patients with advanced non-small cell lung cancer. Tumori. 2012;98:314–319. doi: 10.1177/030089161209800306. [DOI] [PubMed] [Google Scholar]
- 19.Han RX, Liu X, Pan P, Jia YJ, Yu JC. Effectiveness and safety of chemotherapy combined with dendritic cells co-cultured with cytokine-induced killer cells in the treatment of advanced non-small-cell lung cancer: A systematic review and meta-analysis. PLoS One. 2014;9:e108958. doi: 10.1371/journal.pone.0108958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Lin G, Guo ZQ, Zhou ZF, He ZY, Ye YB. Effects of MICA expression on the prognosis of advanced non-small cell lung cancer and the efficacy of CIK therapy. PloS One. 2013;8:e69044. doi: 10.1371/journal.pone.0069044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.González-Cao M. Immunotherapy for lung cancer. Transl Lung Cancer Res. 2015;4:675–677. doi: 10.3978/j.issn.2218-6751.2015.12.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahoney KM, Atkins MB. Prognostic and predictive markers for the new immunotherapies. Oncology 28 Suppl. 2014;3:39–48. [PubMed] [Google Scholar]
- 24.Wang Y, Dai H, Li H, Lv H, Wang T, Fu X, Han W. Growth of human colorectal cancer SW1116 cells is inhibited by cytokine-induced killer cells. Clin Dev Immunol. 2011;2011:621414. doi: 10.1155/2011/621414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gehrmann U, Hiltbrunner S, Georgoudaki AM, Karlsson MC, Näslund TI, Gabrielsson S. Synergistic induction of adaptive antitumor immunity by codelivery of antigen with α-galactosylceramide on exosomes. Cancer Res. 2013;73:3865–3876. doi: 10.1158/0008-5472.CAN-12-3918. [DOI] [PubMed] [Google Scholar]
- 26.Belardelli F, Ferrantini M. Cytokines as a link between innate and adaptive antitumor immunity. Trends Immunol. 2002;23:201–208. doi: 10.1016/S1471-4906(02)02195-6. [DOI] [PubMed] [Google Scholar]
- 27.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu F, Wang ZB, Lu P, Xu ZL, Chen WZ, Zhu H, Jin CB. Activated anti-tumor immunity in cancer patients after high intensity focused ultrasound ablation. Ultrasound Med Biol. 2004;30:1217–1222. doi: 10.1016/j.ultrasmedbio.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Ciernik IF, Berzofsky JA, Carbone DP. Induction of cytotoxic T lymphocytes and antitumor immunity with DNA vaccines expressing single T cell epitopes. J Immunol. 1996;156:2369–2375. [PubMed] [Google Scholar]
- 30.Kirkwood JM, Butterfield LH, Tarhini AA, Zarour H, Kalinski P, Ferrone S. Immunotherapy of cancer in 2012. CA Cancer J Clin. 2012;62:309–335. doi: 10.3322/caac.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li R, Wang C, Liu L, Du C, Cao S, Yu J, Wang SE, Hao X, Ren X, Li H. Autologous cytokine-induced killer cell immunotherapy in lung cancer: A phase II clinical study. Cancer Immunol Immunother. 2012;61:2125–2133. doi: 10.1007/s00262-012-1260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li DP, Li W, Feng J, Chen K, Tao M. Adjuvant chemotherapy with sequential cytokine-induced killer (CIK) cells in stage IB non-small cell lung cancer. Oncol Res. 2015;22:67–74. doi: 10.3727/096504014X14024160459168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhong R, Han B, Zhong H. A prospective study of the efficacy of a combination of autologous dendritic cells, cytokine-induced killer cells, and chemotherapy in advanced non-small cell lung cancer patients. Tumour Biol. 2014;35:987–994. doi: 10.1007/s13277-013-1132-1. [DOI] [PubMed] [Google Scholar]
- 34.Zhong R, Teng J, Han B, Zhong H. Dendritic cells combining with cytokine-induced killer cells synergize chemotherapy in patients with late-stage non-small cell lung cancer. Cancer Immunol Immunother. 2011;60:1497–1502. doi: 10.1007/s00262-011-1060-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Huang SL, Wu YF, Wei J, Bao R, Zhou DH. Expansion of CIK/NK cells from cord blood by using different combinations of stem cell factor, FLT3 ligand and interleukin 2, 7, 15 in vitro. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2004;12:350–354. (In Chinese) [PubMed] [Google Scholar]
- 36.Li Y, Schmidt-Wolf IG, Wu YF, Huang SL, Wei J, Fang J, Huang K, Zhou DH. Optimized protocols for generation of cord blood-derived cytokine-induced killer/natural killer cells. Anticancer Res. 2010;30:3493–3499. [PubMed] [Google Scholar]
- 37.Ritchie RF, Palomaki GE, Neveux LM, Navolotskaia O, Ledue TB, Craig WY. Reference distributions for the negative acute-phase serum protein–s, albumin, transferrin and transthyretin: A practical, simple and clinically relevant approach in a large cohort. J Clin Lab Anal. 1999;13:273–279. doi: 10.1002/(SICI)1098-2825(1999)13:6<273::AID-JCLA4>3.0.CO;2-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Don BR, Kaysen G. Serum albumin: Relationship to inflammation and nutrition. Semin Dial. 2004;17:432–437. doi: 10.1111/j.0894-0959.2004.17603.x. [DOI] [PubMed] [Google Scholar]
- 39.von Meyenfeldt M. Cancer-associated malnutrition: An introduction. Eur J Oncol Nurs. 2005;9(Suppl 2):S35–S38. doi: 10.1016/j.ejon.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: A systematic review of the epidemiological literature. Nutr J. 2010;9:69. doi: 10.1186/1475-2891-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeun JY, Kaysen GA. Factors influencing serum albumin in dialysis patients. Am J Kidney Dis. 1998;32(Suppl 4):S118–S125. doi: 10.1016/S0272-6386(98)70174-X. [DOI] [PubMed] [Google Scholar]
- 42.Siddiqui A, Heinzerling J, Livingston EH, Huerta S. Predictors of early mortality in veteran patients with pancreatic cancer. Am J Surg. 2007;194:362–366. doi: 10.1016/j.amjsurg.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Onate-Ocana LF, Aiello-Crocifoglio V, Gallardo-Rincón D, Herrera-Goepfert R, Brom-Valladares R, Carrillo JF, Cervera E, Mohar-Betancourt A. Serum albumin as a significant prognostic factor for patients with gastric carcinoma. Ann Surg Oncol. 2007;14:381–389. doi: 10.1245/s10434-006-9093-x. [DOI] [PubMed] [Google Scholar]
- 44.Lis CG, Grutsch JF, Vashi PG, Lammersfeld CA. Is serum albumin an independent predictor of survival in patients with breast cancer? JPEN J Parenter Enteral Nutr. 2003;27:10–15. doi: 10.1177/014860710302700110. [DOI] [PubMed] [Google Scholar]
- 45.Seve P, Ray-Coquard I, Trillet-Lenoir V, Sawyer M, Hanson J, Broussolle C, Negrier S, Dumontet C, Mackey JR. Low serum albumin levels and liver metastasis are powerful prognostic markers for survival in patients with carcinomas of unknown primary site. Cancer. 2006;107:2698–2705. doi: 10.1002/cncr.22300. [DOI] [PubMed] [Google Scholar]
- 46.Burkholder B, Huang RY, Burgess R, Luo S, Jones VS, Zhang W, Lv ZQ, Gao CY, Wang BL, Zhang YM, Huang RP. Tumor-induced perturbations of cytokines and immune cell networks. Biochim Biophys Acta. 2014;1845:182–201. doi: 10.1016/j.bbcan.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Souza SL, Da Montalli Assumpção LV, Ward LS. Impact of previous thyroid autoimmune diseases on prognosis of patients with well-differentiated thyroid cancer. Thyroid. 2003;13:491–495. doi: 10.1089/105072503322021160. [DOI] [PubMed] [Google Scholar]
- 48.Krauze MT, Tarhini A, Gogas H, Kirkwood JM. Prognostic significance of autoimmunity during treatment of melanoma with interferon. Semin Immunopathol. 2011;33:385–391. doi: 10.1007/s00281-011-0247-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weber J. Ipilimumab: Controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother. 2009;58:823–830. doi: 10.1007/s00262-008-0653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Braumuller H, Wieder T, Brenner E, Aßmann S, Hahn M, Alkhaled M, Schilbach K, Essmann F, Kneilling M, Griessinger C, et al. T-helper-1-cell cytokines drive cancer into senescence. Nature. 2013;494:361–365. doi: 10.1038/nature11824. [DOI] [PubMed] [Google Scholar]
- 51.Salih HR, Rammensee HG, Steinle A. Cutting edge: Down-regulation of MICA on human tumors by proteolytic shedding. J Immunol. 2002;169:4098–4102. doi: 10.4049/jimmunol.169.8.4098. [DOI] [PubMed] [Google Scholar]
- 52.Ascierto PA, Capone M, Urba WJ, Bifulco CB, Botti G, Lugli A, Marincola FM, Ciliberto G, Galon J, Fox BA. The additional facet of immunoscore: Immunoprofiling as a possible predictive tool for cancer treatment. J Transl Med. 2013;11:54. doi: 10.1186/1479-5876-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]