Fig. 1.
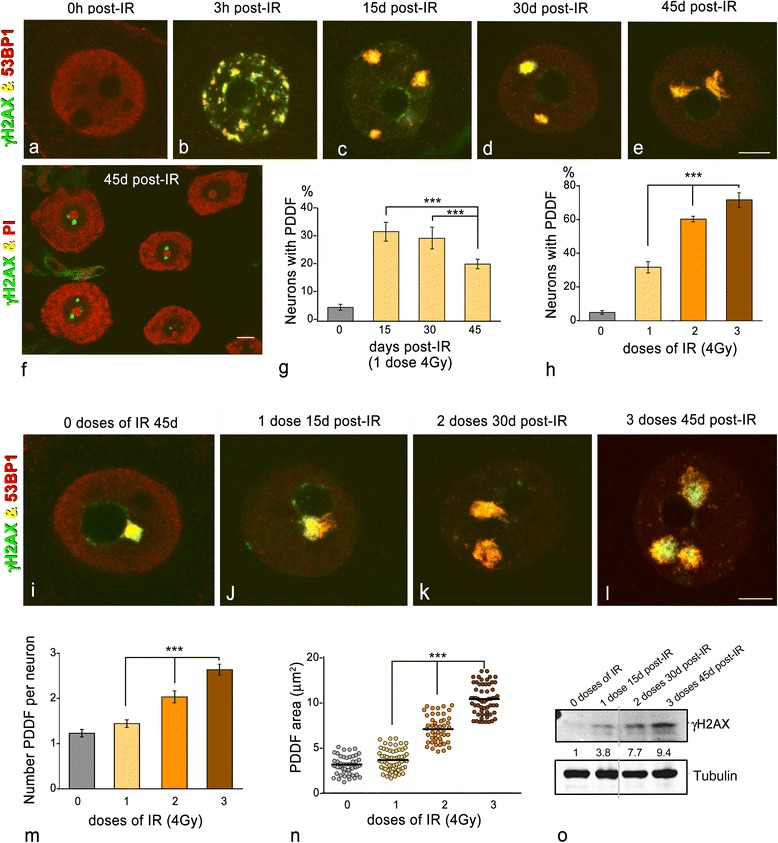
a–e Representative examples of double immunolabeling for γH2AX and 53BP1 in dissociated sensory ganglion neurons from control (a) and irradiated neurons at 3 h, 15d, 30d and 45d pos-IR (b–e). a Control neurons lack of γH2AX signal and exhibit a diffuse nucleoplasmic labeling for 53BP1. b At 3 h post-IR, γH2AX and 53BP1 colocalize in numerous DNA damage foci of variable size and distributed throughout the nucleus, excepting the nucleolus. c–e At 15d, 30d and 45d post-IR, one to three large PDDF appear intensely immunostained for γH2AX and 53BP1. Scale bar: 5 μm. f SGN perikarya immunolabeled for γH2AX and counterstained with propidium iodide (PI) illustrate the preferentially perinucleolar location of PDDF. Scale bar: 10 μm. g Proportion of SGNs containing γH2AX-positive PDDF at 15d, 30d and 45d post-IR, and irradiated with a single dose (4Gy) (data are mean ± SD from three independent experiments, at least 100 neurons per group were counted; ***p < 0.001). h Proportion of SGNs containing γH2AX-positive PDDF following the administration of one, two and three doses of IR, and 15 days after the last treatment. (data are mean ± SD from three independent experiments, at least 100 neurons per group were counted; ***p < 0.001). i–l Double immunolabeling for γH2AX and 53BP1 in SGNs from control (i) and irradiated neurons with one, two or three doses (j–l). Note a spontaneous perinucleolar PDDF in a control neuron and the dose-dependent increase of PDDF in irradiated neurons. Scale bar: 5 μm. (m, n) Mean number per neuron and size of PDDF following the administration of one to three doses of IR. Note the dose-dependent increase in the number and size of foci (data are mean ± SD from three independent experiments; ***p < 0.001). o Western blot analysis of phosphorylated histone H2AX at Ser 139 (γH2AX) in sensory ganglion lysates from controls and irradiated animals (n = 3 animals per group). The expression of γH2AX was induced with IR and its protein levels increased in dose-dependent manner. The expression of alpha-tubulin band was used as a protein loading control, and the fold increase estimated
