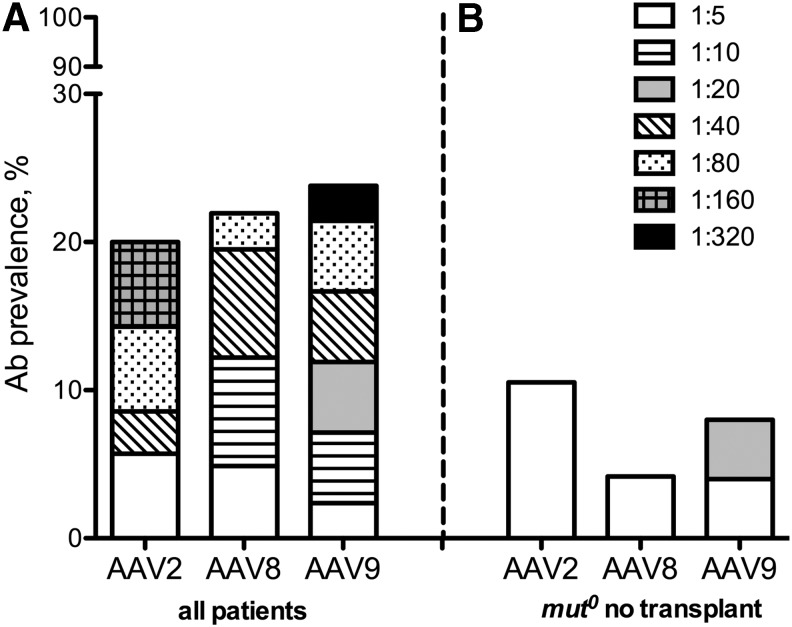
Figure 1.
(A) AAV antibody seroprevalance within the entire MMA cohort (n = 42) that includes both nontransplanted and transplanted (liver, kidney, and/or combined liver–kidney; n = 10) patients. The overall seroprevalance, defined as ≥1:5, is depicted (20–24%) along with the titer distribution of antibodies against AAV2 (n = 35), AAV8 (n = 41), and AAV9 (n = 42) capsids. The seroprevalence is subdivided into titer dilution levels from weak (1:5) NAb titers to strong (1:320). (B) AAV antibody seropositivity in the subgroup of patients with mut0 MMA who were not transplanted (n = 24), which shows low NAb prevalence (4–10%) for the cohort of severe, nontransplanted patients who would be ideal candidates for gene therapy. AAV, adeno-associated viral; Ab, antibody; MMA, methylmalonic acidemia; NAb, neutralizing antibody.