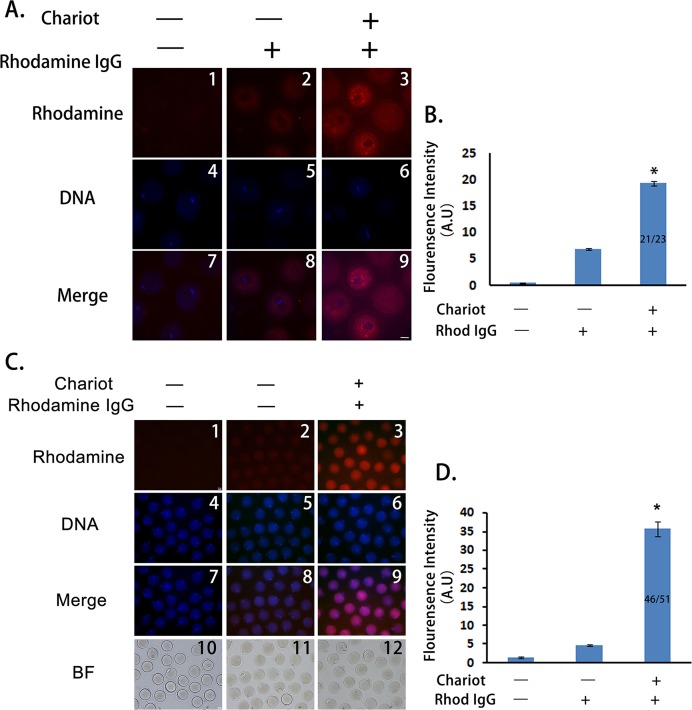
Figure 1. Peptide nanoparticle-encapsulated antibody can effectively enter intact mouse oocytes.
(A) Fluorescence comparison between three different groups at MI stage. A1, A4, A7, control oocytes without any treatment; A2, A5, A8, oocytes were incubated with rhodamine-conjugated rabbit IgG; A3, A6, A9, oocytes were incubated with peptide nanoparticle-encapsulated rhodamine-conjugated rabbit IgG; by line, A1–A3, rhodamine-conjugated rabbit IgG in red; A4–A6, DNA in blue; A7–A9, merge of rhodamine and DNA. (B) Quantification of rhodamine fluorescence of three groups in (A). Fraction in the column is number of oocytes with bright rhodamine signal/number of all examined MI oocytes. (C) Fluorescence comparison between three different groups at MII stage. C1, C4, C7, C10, control oocytes without any treatment; C2, C5, C8, C11, oocytes were incubated with rhodamine-conjugated rabbit IgG; C3, C6, C9, C12, oocytes were incubated with peptide nanoparticle-encapsulated rhodamine-conjugated rabbit IgG; by line, C1–C3, rhodamine-conjugated rabbit IgG in red; C4–C6, DNA in blue; C7–C9, merge of rhodamine and DNA. C10–C12, bright field (BF). (D) Quantification of rhodamine fluorescence of three groups in (C). Fraction in the column is the number of oocytes with bright rhodamine signal/number of all examined MII oocytes. Significant comparisons (p < 0.05) marked with asterisks (*). Scale bar, 20 μm.