Abstract
Alterations of verbal fluency may correlate with deficits of gray matter volume and hemispheric lateralization of language brain regions like the pars triangularis (PT) in schizophrenia. Examining non-psychotic individuals at high genetic risk (HR) for schizophrenia may clarify if these deficits represent heritable trait markers or state dependent phenomena. We assessed adolescent and young adult HR subjects (N=60) and healthy controls (HC; N=42) using verbal fluency tests and Freesurfer to process T1-MRI scans. We hypothesized volumetric and lateralization alterations of the PT and their correlation with verbal fluency deficits. HR subjects had letter verbal fluency deficits (controlling for IQ), left PT deficits (p=.00), (controlling ICV) and reversal of the L>R PT asymmetry noted in HC. Right Heschl’s (p=.00), left supramarginal (p=.00) and right angular gyrii (p=.02) were also reduced in HR subjects. The L>R asymmetry of the Heschl’s gyrus seen in HC was exaggerated and asymmetries of L>R of supramarginal and R>L of angular gyri, seen in HC were attenuated in HR subjects. L>R asymmetry of the PT predicted better verbal fluency across the pooled HR and HC groups. Young relatives of schizophrenia patients have verbal fluency deficits, gray matter volume deficits and reversed asymmetry of the pars triangularis. A reversed structural asymmetry of the PT in HR subjects may impair expressive language abilities leading to verbal f;uency deficits. Volumetric deficits and altered asymmetry in inferior parietal and Heschl’s gyrii may accompany genetic liability to schizophrenia.
Keywords: Schizophrenia, Verbal fluency, Lateralization, Gray matter volume, Pars triangularis
1. Introduction
Schizophrenia is a devastating mental illness involving alterations of language (Crow, 1997b, 2000a, 2004) such as verbal fluency deficits (Goldberg et al., 1998; Riley et al., 2000). Hemispheric lateralization of gray matter (Crow 1997b, 2000a, 2004) may allow segregated language processing (functional lateralization) in right and left hemispheres (Seldon 2005; Warrier et al., 2009). Lateralization in schizophrenia has been shown to correlate with language function (Hoff et al., 1992). Structural (Blackwood et al., 1991; Lee et al., 2007; McCarley et al., 2002; Meisenzahl et al., 2004; O’Donnell et al., 1995) and functional lateralization deficits (Costafreda et al., 2006; Dollfus et al., 2005; Fu et al., 2005; Hirshorn and Thompson-Schill, 2006; Kircher et al., 2009; Lux et al., 2008; Pearlson et al., 1996; Prata et al., 2008; Takizawa et al., 2008; Voets et al., 2006) are associated with language dysfunction and may correlate with verbal fluency deficits in schizophrenia (Antonova et al., 2005; Baare et al., 1999; Costafreda et al., 2006; Crow 2000a, 2004; Dollfus et al., 2005; Fu et al., 2005; Hirshorn and Thompson-Schill, 2006; Kircher et al., 2009; Lux et al., 2008; Pearlson et al., 1996; Prata et al., 2008; Takizawa et al., 2008; Voets et al., 2006).
The anterior language regions involving the inferior frontal gyri facilitate expressive aspects of language (Hashimoto et al., 2009; Matsumoto et al., 2004; Price 2000; Shalom and Poeppel 2008) and may mediate verbal fluency (Costafreda et al., 2006; Fu et al., 2005; Hirshorn and Thompson-Schill 2006; Kircher et al., 2009; Lux et al., 2008; Pearlson et al., 1996; Prata et al., 2008; Takizawa et al., 2008; Voets et al., 2006). Altered lateralization of structure (Kawasaki et al., 2008; Selemon et al., 2003; Venkatasubramanian et al., 2008; Wisco et al., 2007) and function (Dollfus et al., 2005) for the pars triangularis (PT), a sub-region of the inferior frontal gyrus (Kawasaki et al., 2008), is noted in schizophrenia.
Language area asymmetry deficits in schizophrenia (Crow 1997a, 2000a; Hallett et al., 1986) may have genetic bases (Crow 2000a,b), and may manifest in genetically predisposed subjects. Volumetric and lateralization alterations of the PT noted in patients (Selemon et al., 2003; Wisco et al., 2007) may also occur in relatives of patients and underlie verbal fluency deficits in this population (Gilvarry et al., 2001; Keefe et al., 1994; Klosterkotter et al., 2001; Lencz et al., 2006; Simon et al., 2007). Studies in those at genetic risk for schizophrenia independently show verbal fluency deficits (Gilvarry et al., 2001; Keefe et al., 1994; Klosterkotter et al., 2001; Lencz et al., 2006; Simon et al., 2007) and alterations of language area lateralization (Sharma et al., 1999) but it is unclear if these phenomena are correlated.
In this study, we primarily assessed adolescent and young adult relatives of patients on verbal fluency, gray matter volume of the PT, its structural lateralization and correlation of verbal fluency with PT lateralization.
2. Experimental/materials and methods
Participants
Sixty first and second-degree young (9 to 25 years) relatives of schizophrenia patients (HR) and forty-two age- and gender-matched healthy comparison subjects from an ongoing study at the University of Pittsburgh participated in this study. HR subject group were composed of forty-seven first-degree relatives and thirteen second-degree relatives. Individuals with IQ>80, no lifetime evidence of a psychotic disorder, antipsychotic use, substance use, or significant neurological or medical condition were included. All participants signed an informed consent after full explanation of the study. The study was approved by the University of Pittsburgh Institutional Review Board.
Language function
Verbal fluency measures were assessed using Multilingual Aphasia Examination Manual (Benton and Hamscher, 1978) including a letter task (number of words generated in 20 s that start on C, F and L alphabets) and a category task (e.g. names of animals, fruits and vegetables). Total verbal fluency scores were assessed.
Image acquisition
MRI scans were obtained on subjects using a GE 1.5 T whole body scanner (GE Medical Systems, Milwaukee, Wisconsin). The detailed scanning protocol has been described in an earlier publication (Gilbert et al. 2001). Briefly, the scans were three-dimension spoiled gradient recalled (SPGR), acquired in a steady-state pulse sequence (124 coronal slices, 1.5 mm cortical thickness, TE=5 ms, TR=25 ms, acquisition matrix=256×192, FOV=24 cm, flip angle 40°). Images with motion artifacts were not included in the study.
Image analysis
T1-images were processed using Freesurfer. Freesurfer has three automated stages (Segonne et al., 2004), each followed by manual image editing by an experienced neuroanatomist. The first stage performs skull stripping, motion correction and gray-white segmentation (Fischl et al., 2002; Han et al., 2004; Han et al., 2002). This is followed by an automated parcellation of the cortex based on gyral anatomical landmarks and gray matter volume measurements of the parcellated regions (Desikan et al., 2006; Fischl et al., 2004); a method shown to be valid and reliable with manual tracing. Freesurfer has been used to study the brain morphology of schizophrenia patients and their relatives (Kuperberg et al., 2003; Nesvag et al., 2008; Voets et al., 2008), is reliable and accurate with manual and automated methods (Desikan et al., 2006; Fischl et al., 2002; Fischl et al., 2004; Pengas et al., 2009; Tae et al., 2008) and is robust to anatomical alterations noted in schizophrenia (Boos et al., 2007).
The anterior language regions, which include the PT, facilitate expressive aspects of language (Hashimoto et al., 2009; Matsumoto et al., 2004; Price 2000; Shalom and Poeppel 2008) and may mediate verbal fluency (Costafreda et al., 2006; Fu et al., 2005; Hirshorn and Thompson-Schill 2006; Kircher et al., 2009; Lux et al., 2008; Pearlson et al., 1996; Prata et al., 2008; Takizawa et al., 2008; Voets et al., 2006). The PT was primarily assessed as its alterations have been widely demonstrated (Gaser et al., 2004; Kawasaki et al., 2008; Selemon et al., 2003; Spalletta et al., 2003; Venkatasubramanian et al., 2008; Wisco et al., 2007) in schizophrenia patients and may contribute to their verbal fluency deficits (Crow 2000a, b). We hypothesized that lateralization of the PT is altered and correlates abnormally with verbal fluency in relatives. Pars opercularis, another sub-region of the inferior frontal gyrus involved in language processing (Hsieh et al., 2001; Matsumoto et al., 2004) has not been implicated in schizophrenia and hence was not assessed (Shenton et al., 2001).
The posterior language regions of the planum temporale, Heschl’s gyrus and inferior parietal lobule (Costafreda et al., 2006; Fu et al., 2005; Phelps et al., 1997; Price, 2000; Shalom and Poeppel 2008) perform receptive language functions (Aboitiz and Garcia 1997; Catani et al., 2005; Shenton et al., 2001) and hence may not be directly involved in verbal fluency. These were secondarily assessed given their role in language function (Price 2000; Shalom and Poeppel 2008) and that they are altered in schizophrenia (Buchanan et al., 2004; Erwin and Rosenbaum 1979; Gaser et al., 2004; Goldstein et al., 1999; Hulshoff Pol et al., 2001; Kawasaki et al., 2008; Matsumoto et al., 2001; Shenton et al., 2001; Zhou et al., 2007). The planum was not assessed as Freesurfer does not parcellate this area separately.
Statistical methods
Data was rank transformed before being analyzed using parametric methods as some measures were not normally distributed [Shapiro-Wilk’s test (W statistic, p>0.05)]. Verbal fluency scores were compared between groups using ANCOVA controlling IQ. Gray matter volumes for each side were first compared between groups using ANCOVA controlling for ICV and age. Bonferroni threshold of p =.006[0.05/8] was used to control the experiment-wise error of these eight [2 sides×4 regions] comparisons. Volumes were also compared using repeated-
measures ANCOVA with side as the within-subject factor and study group as between-subject factor to assess group×side interactions, controlling ICV and age. Laterality indices (laterality index=Left volume-right volume/Left volume+ right volume) were compared between groups using ANCOVA, controlling age. Bonferroni threshold of p=.012 [0.05/4] was applied to each of these sets of four comparisons. Partial correlations (two tailed, controlling for age and IQ) between verbal fluency scores and PT laterality index were performed pooled over control and HR subjects and also separately for each group.
3. Results
Healthy controls had higher IQ scores than HR [t=4.51, p=0.000] and did not differ from HR on age [controls (16.6, 4.5), HR (15.4, 3.6), (Mean, SD, years), t=1.79, p=0.1), handedness (Chi-square=0.1, p=0.7), race (Chi-square=0.33, p=0.57) and gender (controls: 42% males, HR: 53% males, Chi-square=0.16, p=0.20).
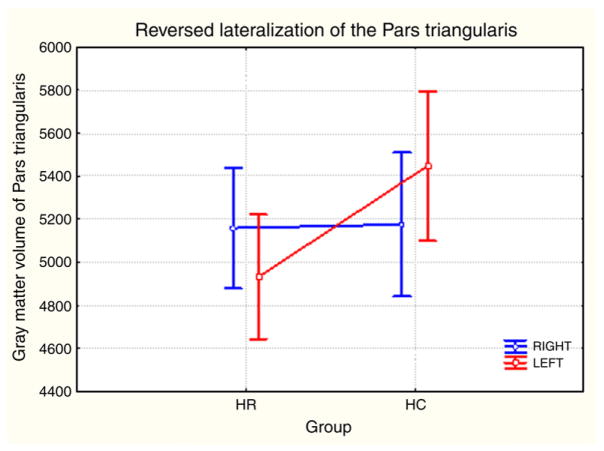
HR subjects performed poorly on the letter but not category verbal fluency test [total verbal fluency score F(1,99)= 5.01 p=0.027, letter verbal fluency score F(1,99)=4.89, p=0.029, category verbal fluency score, p=0.193] after controlling IQ. HR subjects had smaller left PT [F(1,98)= 8.04 p =0.005] and reversal of the L>R lateralization of PT seen in HC after Bonferroni corrections (see Table 1 and Fig. 1).
Table 1.
Repeated-measures ANCOVAs showing group×side interaction effects and laterality index comparisons between HC and HR.
| Group by side interaction F(1,98), p | Across group laterality index comparison F(1,98), p | Direction of lateralization | Lateralization in HR compared to HC | |
|---|---|---|---|---|
| Pars triangularis | 7.78, .008 a | 7.15, .008 a | HC :L>R | Reversed |
| HC :L<R | ||||
| Heschl’s gyrus | 2.23, .137 | .6, .030 | HC :L>R | Exaggerated |
| HC :L≫R | ||||
| Angular gyrus | 10.31, .002 a | 7.25, .008 a | HC :L≪R | Attenuated |
| HC :L<R | ||||
| Supramarginal gyrus | 3.57, .062 | 7.20, .009 a | HC :L≫R | Attenuated |
| HC :L>R |
Bonferroni threshold of p=.0125 (.05/4) was used to separately correct repeated-measures ANCOVAs and laterality comparisons for four comparisons for experiment-wise error.
Survived the Bonferroni threshold.
Fig. 1.
Reversed lateralization of the gray matter volumes of the pars triangularis.
HR subjects had deficits of left supramarginal [F(1,98)= 11.05 p=0.001], right Heschl’s [F(1,98)=8.70, p=0.004] and right angular gyrii [F(1,98)=6.48, p=0.012] which survived the Bonferroni threshold. The L>R lateralization seen in HC for the supramarginal gyrus was attenuated in HR. The lateralization of the angular gyrus was R>L in HC which was attenuated in HR. The Heschl’s gyral L>R asymmetry in the HC was exaggerated in HR subjects (see Table 1).
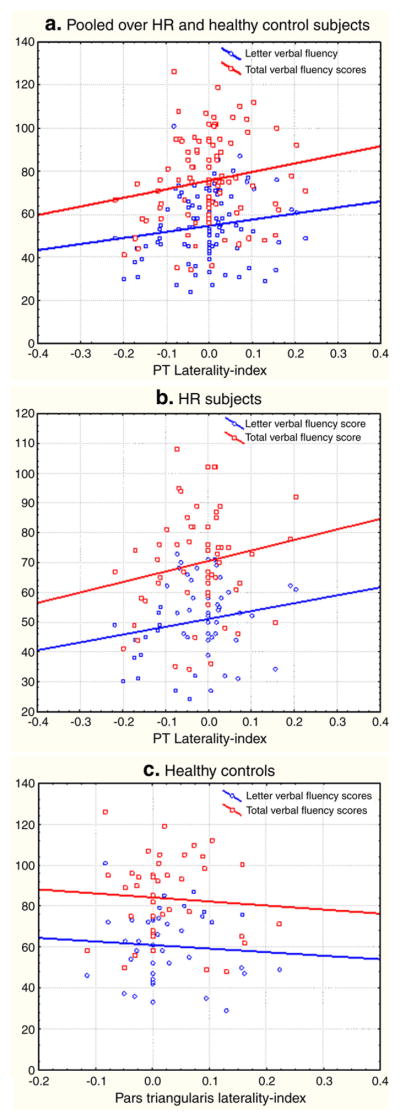
Letter verbal fluency scores positively correlated with the lateralization index of the PT when correlations were performed pooled over HR and control groups (R=0.22, p=0.026). Total verbal fluency (R=0.24, p=0.019), but not category verbal fluency scores (R=0.16, p =0.12) also positively correlated with the lateralization index of the PT. These results suggest that leftward structural lateralization of the pars triangularis predicts better verbal fluency. Within-group correlations showed that the PT lateralization index in HR subjects correlated with letter verbal fluency (R=0.29, p = 0.027) and total verbal fluency scores (R = 0.30, p=0.025). PT lateralization index in healthy controls did not correlate with letter(R = 0.04, p = 0.7) and total (R=0.03, p=0.8) verbal fluency scores (see Fig. 2).
Fig. 2.
a, b and c. Scatterplots of total and letter verbal fluency scores on the Y-axis and pars triangularis laterality index on the X-axis. Three plots for pooled (2a) and within-group correlation analyses (2b and 2c) are shown. HR subjects show correlations between verbal fluency and laterality but these relations were not noted for healthy controls.
Fig. 2a, b and c. Scatterplots of total and letter verbal fluency scores on the Y-axis and pars triangularis laterality index on the X-axis. Three plots for pooled and within-group correlation analyses are shown. HR subjects show correlations between verbal fluency and laterality but these relations were not noted for healthy controls.
All the above results survived exclusion of left handed and ambidextrous subjects (two subjects in HC and two in HR groups), except for those for the Heschl’s gyral laterality. Heschl’s gyral laterality difference between groups was significant after the exclusion of left handed and ambidextrous subjects at a trend level (F(1,94)=3.01, p=0.07). All volumetric reductions and laterality index alterations observed in HR subjects maintained statistical significance after performing the same analyses comparing only first-degree relatives (n=47) to healthy control subjects, except for the supramarginal gyral laterality attenuation in HR which became non-significant [F(1,85)=2.21, p=0.19]. The correlation between the PT laterality index and letter and total verbal fluency noted in HR subjects lost significance for the first-degree relative group, possibly due to reduced power.
4. Discussion
Deficits in letter verbal fluency, gray matter volume reductions and reversal of asymmetry of PT were seen in adolescent and young adult HR subjects suggesting that verbal fluency deficits in HR subjects may be related to abnormal lateralization of the anterior language areas.
Verbal fluency deficits were noted in the letter verbal fluency but not in category verbal fluency domain, consistent with previous findings of reduction in letter verbal fluency but not category in relatives of schizophrenia patients (Egan et al., 2001; Keefe et al., 1994). This may suggest more severe alterations of the anterior language regions which primarily sub serve letter fluency (Baldo et al., 2006; Kircher et al., 2008; Mummery et al., 1996) compared to alterations of the hippocampus and posterior language regions which mediate category fluency. The leftward asymmetry of the PT noted in control subjects was reversed in HR subjects who showed a rightward lateralized PT. Higher letter verbal fluency scores were positively predicted by increased leftward lateralization of the PT suggesting a leftward lateralized functional lateralization for letter verbal fluency in HR subjects. Although a correlation of verbal fluency with increased language area lateralization has been posited for both healthy and HR subjects (Crow, 2000a), we unexpectedly found this relation only for HR subjects. A rightward lateralized (reversed) structural asymmetry of the PT in HR subjects may compromise verbal fluency by interfering with the leftward lateralized functional lateralization. These findings imply the importance of the coherence of structural and functional lateralization for verbal fluency. Functional MRI studies reveal abnormally lateralized BOLD responses of the inferior frontal gyrus during language tasks in patients (Elvevag et al., 2001; Gourovitch et al., 2000; Ragland et al., 2008; Schaufelberger et al., 2005). A lateralized BOLD response may be caused by a lateralized oxygen requirement (Bennett et al., 2008; Lilja et al., 2006) which may reflect structural lateralization and not necessarily a right lateralized preference of processing per se. Studies of functional laterality must control for structural asymmetry. Alterations of white-matter, cortical folding, cortical thickness (Gaser et al., 2004; Kawasaki et al., 2008; Selemon et al., 2003; Spalletta et al., 2003; Venkatasubramanian et al., 2008; Wisco et al., 2007) and functional lateralization (Dollfus et al., 2005) of the PT have been noted in patients but are as yet unexplored in their relatives.
HR subjects also had deficits of the left supramarginal right angular and right Heschl’s gyrii, posterior language regions as yet unexplored in relatives of patients. The L>R lateralization of the supramarginal gyrus and R>L lateralization of the angular gyrus noted in HC were attenuated in HR subjects. The Heschl’s gyral L>R asymmetry in the HC was exaggerated (i.e. L≫R) in HR subjects. Gray matter alterations have been observed in schizophrenia patients in the supramarginal gyrus (Gaser et al., 2004) albeit inconsistently (Buchanan et al., 2004; Goldstein et al., 1999; Hulshoff Pol et al., 2001). A rightward [R>L] asymmetry in the angular gyrus has been reported in chronic (Niznikiewicz et al., 2000) and first episode patients (Nierenberg et al., 2005). The Heschl’s gyrus may show leftward (Kasai et al., 2003) or bilateral (Hirayasu et al., 2000; Yamasue et al., 2004) or no volumetric deficits in patients (Cotter et al., 2004; Yamasaki et al., 2007). In line with previous studies, our findings of verbal fluency deficits and coincident altered lateralization of language areas in young individuals genetically predisposed to schizophrenia implicate genetic liability (Crow, 2000a) and early intra-uterine events (Sommer et al., 2002; Spaniel et al., 2007) in causing alterations of cerebral lateralization and accompanying language dysfunction. Structural lateralization of language areas may enable the “compartmentalized” language processing which involves relegating abstract language processing to the right and motor processing to the left hemisphere (Crow, 2004). Disruptions of structural lateralization effect a breach of this hemispheric segregation of language processing possibly causing language dysfunction in schizophrenia patients (Crow, 2004). Auditory verbal hallucinations (AVH) (Gross and Huber, 2008; Thorup et al., 2007) may share a common neurological substrate with verbal fluency deficits. Altered functional and structural lateralization of language areas have may correlate with severity of AVH (Crow, 2000a; Shapleske et al., 2001; Sommer et al., 2001; Zhang et al., 2008). Verbal fluency deficits may be potential cognitive markers of future auditory–verbal hallucinations and of schizophrenia (Klosterkotter et al., 2001; Lencz et al., 2006).
Our study is limited by the relatively small sample size, assessment of both first and second degree relatives (which may have diluted the observed effect by reducing the degree of familial genetic risk), and lack of data on receptive language (Portocarrero et al., 2007). Structural asymmetry may not necessarily imply functional lateralization and suggests the need for confirmatory functional imaging studies. Notwithstanding these limitations, most of the volumetric and lateralization deficits revealed by this study survived the conservative (Lin, 2005) Bonferroni threshold suggesting these to be critical alterations in those at a familial diathesis for schizophrenia. The study demonstrates a relation between verbal fluency deficits and lateralization abnormalities of language areas in young relatives of schizophrenia patients.
Acknowledgments
Role of funding source
National Institute of Mental Health (MH 64023 and 01180 to MK); National Alliance for Research on Schizophrenia and Depression (Independent Investigator award to MK); National Alliance for Research on Schizophrenia and Depression and General Clinical Research Center (GCRC) (M01 RR00056 to MK).
We would like to acknowledge the role of NIMH in funding this study.
Footnotes
Disclosure/conflict of interest statement
This research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
T.S.B conducted the statistical analyses, contributed to the data interpretation and wrote the first draft. M.S.K was the principal investigator, conducted clinical assessments, planned the overall design of the study, revised the first draft and supervised the statistical analyses. R.P, K.P., and S.E contributed to data analyses and interpretation. A.F and S.K analyzed the imaging data. D.M and D.D supervised clinical assessments. V.D helped with planning this study.
References
- Aboitiz F, Garcia VR. The evolutionary origin of the language areas in the human brain. A neuroanatomical perspective. Brain Res Brain Res Rev. 1997;25(3):381–396. doi: 10.1016/s0165-0173(97)00053-2. [DOI] [PubMed] [Google Scholar]
- Antonova E, Kumari V, Morris R, Halari R, Anilkumar A, Mehrotra R, Sharma T. The relationship of structural alterations to cognitive deficits in schizophrenia: a voxel-based morphometry study. Biol Psychiatry. 2005;58(6):457–467. doi: 10.1016/j.biopsych.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Baare WF, Hulshoff Pol HE, Hijman R, Mali WP, Viergever MA, Kahn RS. Volumetric analysis of frontal lobe regions in schizophrenia: relation to cognitive function and symptomatology. Biol Psychiatry. 1999;45(12):1597–1605. doi: 10.1016/s0006-3223(98)00266-2. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Schwartz S, Wilkins D, Dronkers NF. Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. J Int Neuropsychol Soc. 2006;12(6):896–900. doi: 10.1017/S1355617706061078. [DOI] [PubMed] [Google Scholar]
- Bennett MR, Farnell L, Gibson WG. Origins of the BOLD changes due to synaptic activity at astrocytes abutting arteriolar smooth muscle. J Theor Biol. 2008;252(1):123–130. doi: 10.1016/j.jtbi.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Benton A, Hamscher K. Multilingual Aphasia Examination Manual (revised)(1978) Iowa City. Iowa: University of Iowa; 1978. [Google Scholar]
- Blackwood DH, Young AH, McQueen JK, Martin MJ, Roxborough HM, Muir WJ, St Clair DM, Kean DM. Magnetic resonance imaging in schizophrenia: altered brain morphology associated with P300 abnormalities and eye tracking dysfunction. Biol Psychiatry. 1991;30(8):753–769. doi: 10.1016/0006-3223(91)90232-b. [DOI] [PubMed] [Google Scholar]
- Boos HB, Aleman A, Cahn W, Hulshoff Pol H, Kahn RS. Brain volumes in relatives of patients with schizophrenia: a meta-analysis. Arch Gen Psychiatry. 2007;64(3):297–304. doi: 10.1001/archpsyc.64.3.297. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Francis A, Arango C, Miller K, Lefkowitz DM, McMahon RP, Barta PE, Pearlson GD. Morphometric assessment of the heteromodal association cortex in schizophrenia. Am J Psychiatry. 2004;161(2):322–331. doi: 10.1176/appi.ajp.161.2.322. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57(1):8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Fu CH, Lee L, Everitt B, Brammer MJ, David AS. A systematic review and quantitative appraisal of fMRI studies of verbal fluency: role of the left inferior frontal gyrus. Hum Brain Mapp. 2006;27(10):799–810. doi: 10.1002/hbm.20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Frangou S, Hudson L, Landau S. Cell density and cortical thickness in Heschl’s gyrus in schizophrenia, major depression and bipolar disorder. Br J Psychiatry. 2004;185:258–259. doi: 10.1192/bjp.185.3.258. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Is schizophrenia the price that Homo sapiens pays for language? Schizophr Res. 1997a;28(2–3):127–141. doi: 10.1016/s0920-9964(97)00110-2. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Schizophrenia as failure of hemispheric dominance for language. Trends Neurosci. 1997b;20(8):339–343. doi: 10.1016/s0166-2236(97)01071-0. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Invited commentary on: functional anatomy of verbal fluency in people with schizophrenia and those at genetic risk. The genetics of asymmetry and psychosis. Br J Psychiatry. 2000a;176:61–63. doi: 10.1192/bjp.176.1.61. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Schizophrenia as the price that homo sapiens pays for language: a resolution of the central paradox in the origin of the species. Brain Res Brain Res Rev. 2000b;31(2–3):118–129. doi: 10.1016/s0165-0173(99)00029-6. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Auditory hallucinations as primary disorders of syntax: an evolutionary theory of the origins of language. Cogn Neuropsychiatry. 2004;9(1–2):125–145. doi: 10.1080/13546800344000192. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dollfus S, Razafimandimby A, Delamillieure P, Brazo P, Joliot M, Mazoyer B, Tzourio-Mazoyer N. Atypical hemispheric specialization for language in right-handed schizophrenia patients. Biol Psychiatry. 2005;57(9):1020–1028. doi: 10.1016/j.biopsych.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Gscheidle T, Weirich M, Rawlings R, Hyde TM, Bigelow L, Weinberger DR. Relative risk for cognitive impairments in siblings of patients with schizophrenia. Biol Psychiatry. 2001;50(2):98–107. doi: 10.1016/s0006-3223(01)01133-7. [DOI] [PubMed] [Google Scholar]
- Elvevag B, Weinstock DM, Akil M, Kleinman JE, Goldberg TE. A comparison of verbal fluency tasks in schizophrenic patients and normal controls. Schizophr Res. 2001;51(2–3):119–126. doi: 10.1016/s0920-9964(00)00053-0. [DOI] [PubMed] [Google Scholar]
- Erwin BJ, Rosenbaum G. Parietal lobe syndrome and schizophrenia: comparison of neuropsychological deficits. J Abnorm Psychol. 1979;88(3):234–241. doi: 10.1037//0021-843x.88.3.234. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fu CH, Suckling J, Williams SC, Andrew CM, Vythelingum GN, McGuire PK. Effects of psychotic state and task demand on prefrontal function in schizophrenia: an fMRI study of overt verbal fluency. Am J Psychiatry. 2005;162(3):485–494. doi: 10.1176/appi.ajp.162.3.485. [DOI] [PubMed] [Google Scholar]
- Gaser C, Nenadic I, Volz HP, Buchel C, Sauer H. Neuroanatomy of “hearing voices”: a frontotemporal brain structural abnormality associated with auditory hallucinations in schizophrenia. Cereb Cortex. 2004;14(1):91–96. doi: 10.1093/cercor/bhg107. [DOI] [PubMed] [Google Scholar]
- Gilbert AR, Rosenberg DR, Harenski K, Spencer S, Sweeney JA, Keshavan MS. Thalamic volumes in patients with first-episode schizophrenia. Am J Psychiatry. 2001;158(4):618–624. doi: 10.1176/appi.ajp.158.4.618. [DOI] [PubMed] [Google Scholar]
- Gilvarry CM, Russell A, Jones P, Sham P, Hemsley D, Murray RM. Verbal fluency in patients with schizophrenia and affective psychoses and their first-degree relatives. Psychol Med. 2001;31(4):695–704. doi: 10.1017/s0033291701003816. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Aloia MS, Gourovitch ML, Missar D, Pickar D, Weinberger DR. Cognitive substrates of thought disorder, I: the semantic system. Am J Psychiatry. 1998;155(12):1671–1676. doi: 10.1176/ajp.155.12.1671. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Goodman JM, Seidman LJ, Kennedy DN, Makris N, Lee H, Tourville J, Caviness VS, Jr, Faraone SV, Tsuang MT. Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Arch Gen Psychiatry. 1999;56(6):537–547. doi: 10.1001/archpsyc.56.6.537. [DOI] [PubMed] [Google Scholar]
- Gourovitch ML, Kirkby BS, Goldberg TE, Weinberger DR, Gold JM, Esposito G, Van Horn JD, Berman KF. A comparison of rCBF patterns during letter and semantic fluency. Neuropsychology. 2000;14(3):353–360. doi: 10.1037//0894-4105.14.3.353. [DOI] [PubMed] [Google Scholar]
- Gross G, Huber G. Psychopathology of schizophrenia and brain imaging. Fortschr Neurol Psychiatr. 2008;76(Suppl 1):S49–S56. doi: 10.1055/s-2008-1038152. [DOI] [PubMed] [Google Scholar]
- Hallett S, Quinn D, Hewitt J. Defective interhemispheric integration and anomalous language lateralization in children at risk for schizophrenia. J Nerv Ment Dis. 1986;174(7):418–427. doi: 10.1097/00005053-198607000-00006. [DOI] [PubMed] [Google Scholar]
- Han X, Pham DL, Tosun D, Rettmann ME, Xu C, Prince JL. CRUISE: cortical reconstruction using implicit surface evolution. Neuroimage. 2004;23(3):997–1012. doi: 10.1016/j.neuroimage.2004.06.043. [DOI] [PubMed] [Google Scholar]
- Han X, Xu C, Braga-Neto U, Prince JL. Topology correction in brain cortex segmentation using a multiscale, graph-based algorithm. IEEE Trans Med Imaging. 2002;21(2):109–121. doi: 10.1109/42.993130. [DOI] [PubMed] [Google Scholar]
- Hashimoto RI, Lee K, Preus A, McCarley RW, Wible CG. An fMRI study of functional abnormalities in the verbal working memory system and the relationship to clinical symptoms in chronic schizophrenia. Cereb Cortex. 2009 doi: 10.1093/bhp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayasu Y, McCarley RW, Salisbury DF, Tanaka S, Kwon JS, Frumin M, Snyderman D, Yurgelun-Todd D, Kikinis R, Jolesz FA, Shenton ME. Planum temporale and Heschl gyrus volume reduction in schizophrenia: a magnetic resonance imaging study of first-episode patients. Arch Gen Psychiatry. 2000;57(7):692–699. doi: 10.1001/archpsyc.57.7.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshorn EA, Thompson-Schill SL. Role of the left inferior frontal gyrus in covert word retrieval: neural correlates of switching during verbal fluency. Neuropsychologia. 2006;44(12):2547–2557. doi: 10.1016/j.neuropsychologia.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Hoff AL, Riordan H, O’Donnell D, Stritzke P, Neale C, Boccio A, Anand AK, DeLisi LE. Anomalous lateral sulcus asymmetry and cognitive function in first-episode schizophrenia. Schizophr Bull. 1992;18(2):257–272. doi: 10.1093/schbul/18.2.257. [DOI] [PubMed] [Google Scholar]
- Hsieh L, Gandour J, Wong D, Hutchins GD. Functional heterogeneity of inferior frontal gyrus is shaped by linguistic experience. Brain Lang. 2001;76(3):227–252. doi: 10.1006/brln.2000.2382. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Mandl RC, van Haren NE, Koning H, Collins DL, Evans AC, Kahn RS. Focal gray matter density changes in schizophrenia. Arch Gen Psychiatry. 2001;58(12):1118–1125. doi: 10.1001/archpsyc.58.12.1118. [DOI] [PubMed] [Google Scholar]
- Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Onitsuka T, Spencer MH, Yurgelun-Todd DA, Kikinis R, Jolesz FA, McCarley RW. Progressive decrease of left Heschl gyrus and planum temporale gray matter volume in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2003;60(8):766–775. doi: 10.1001/archpsyc.60.8.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Suzuki M, Takahashi T, Nohara S, McGuire PK, Seto H, Kurachi M. Anomalous cerebral asymmetry in patients with schizophrenia demonstrated by voxel-based morphometry. Biol Psychiatry. 2008;63(8):793–800. doi: 10.1016/j.biopsych.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Silverman JM, Roitman SE, Harvey PD, Duncan MA, Alroy D, Siever LJ, Davis KL, Mohs RC. Performance of nonpsychotic relatives of schizophrenic patients on cognitive tests. Psychiatry Res. 1994;53(1):1–12. doi: 10.1016/0165-1781(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Kircher T, Krug A, Markov V, Whitney C, Krach S, Zerres K, Eggermann T, Stocker T, Shah NJ, Treutlein J, Nothen MM, Becker T, Rietschel M. Genetic variation in the schizophrenia-risk gene neuregulin 1 correlates with brain activation and impaired speech production in a verbal fluency task in healthy individuals. Hum Brain Mapp. 2009 doi: 10.1002/hbm.20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher T, Whitney C, Krings T, Huber W, Weis S. Hippocampal dysfunction during free word association in male patients with schizophrenia. Schizophr Res. 2008;101(1–3):242–255. doi: 10.1016/j.schres.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Klosterkotter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry. 2001;58(2):158–164. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SC, van der Kouwe AJ, Salat DH, Dale AM, Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60(9):878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Lee H, Devlin JT, Shakeshaft C, Stewart LH, Brennan A, Glensman J, Pitcher K, Crinion J, Mechelli A, Frackowiak RS, Green DW, Price CJ. Anatomical traces of vocabulary acquisition in the adolescent brain. J Neurosci. 2007;27(5):1184–1189. doi: 10.1523/JNEUROSCI.4442-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencz T, Smith CW, McLaughlin D, Auther A, Nakayama E, Hovey L, Cornblatt BA. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol Psychiatry. 2006;59(9):863–871. doi: 10.1016/j.biopsych.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Lilja J, Endo T, Hofstetter C, Westman E, Young J, Olson L, Spenger C. Blood oxygenation level-dependent visualization of synaptic relay stations of sensory pathways along the neuroaxis in response to graded sensory stimulation of a limb. J Neurosci. 2006;26(23):6330–6336. doi: 10.1523/JNEUROSCI.0626-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DY. An efficient Monte Carlo approach to assessing statistical significance in genomic studies. Bioinformatics. 2005;21(6):781–787. doi: 10.1093/bioinformatics/bti053. [DOI] [PubMed] [Google Scholar]
- Lux S, Keller S, Mackay C, Ebers G, Marshall JC, Cherkas L, Rezaie R, Roberts N, Fink GR, Gurd JM. Crossed cerebral lateralization for verbal and visuo-spatial function in a pair of handedness discordant monozygotic twins: MRI and fMRI brain imaging. J Anat. 2008;212(3):235–248. doi: 10.1111/j.1469-7580.2008.00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Simmons A, Williams S, Hadjulis M, Pipe R, Murray R, Frangou S. Superior temporal gyrus abnormalities in early-onset schizophrenia: similarities and differences with adult-onset schizophrenia. Am J Psychiatry. 2001;158(8):1299–1304. doi: 10.1176/appi.ajp.158.8.1299. [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Nair DR, LaPresto E, Najm I, Bingaman W, Shibasaki H, Luders HO. Functional connectivity in the human language system: a cortico-cortical evoked potential study. Brain. 2004;127(Pt 10):2316–2330. doi: 10.1093/brain/awh246. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Salisbury DF, Hirayasu Y, Yurgelun-Todd DA, Tohen M, Zarate C, Kikinis R, Jolesz FA, Shenton ME. Association between smaller left posterior superior temporal gyrus volume on magnetic resonance imaging and smaller left temporal P300 amplitude in first-episode schizophrenia. Arch Gen Psychiatry. 2002;59(4):321–331. doi: 10.1001/archpsyc.59.4.321. [DOI] [PubMed] [Google Scholar]
- Meisenzahl EM, Frodl T, Muller D, Schmitt G, Gallinat J, Zetzsche T, Marcuse A, Juckel G, Leinsinger G, Hahn K, Moller HJ, Hegerl U. Superior temporal gyrus and P300 in schizophrenia: a combined ERP/structural magnetic resonance imaging investigation. J Psychiatr Res. 2004;38(2):153–162. doi: 10.1016/s0022-3956(03)00078-5. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Hodges JR, Wise RJ. Generating ‘tiger’ as an animal name or a word beginning with T: differences in brain activation. Proc Biol Sci. 1996;263(1373):989–995. doi: 10.1098/rspb.1996.0146. [DOI] [PubMed] [Google Scholar]
- Nesvag R, Lawyer G, Varnas K, Fjell AM, Walhovd KB, Frigessi A, Jonsson EG, Agartz I. Regional thinning of the cerebral cortex in schizophrenia: effects of diagnosis, age and antipsychotic medication. Schizophr Res. 2008;98(1–3):16–28. doi: 10.1016/j.schres.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Nierenberg J, Salisbury DF, Levitt JJ, David EA, McCarley RW, Shenton ME. Reduced left angular gyrus volume in first-episode schizophrenia. Am J Psychiatry. 2005;162(8):1539–1541. doi: 10.1176/appi.ajp.162.8.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niznikiewicz M, Donnino R, McCarley RW, Nestor PG, Iosifescu DV, O’Donnell B, Levitt J, Shenton ME. Abnormal angular gyrus asymmetry in schizophrenia. Am J Psychiatry. 2000;157(3):428–437. doi: 10.1176/appi.ajp.157.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell BF, Shenton ME, McCarley RW, Faux SF, Kikinis R, Nestor PG, Jolesz FA. Conjoint left asymmetry of auditory P300 voltage and MRI volume of posterior superior temporal gyrus in schizophrenia: a quantitative evaluation. Electroencephalogr Clin Neurophysiol Suppl. 1995;44:387–394. [PubMed] [Google Scholar]
- Pearlson GD, Petty RG, Ross CA, Tien AY. Schizophrenia: a disease of heteromodal association cortex? Neuropsychopharmacology. 1996;14(1):1–17. doi: 10.1016/S0893-133X(96)80054-6. [DOI] [PubMed] [Google Scholar]
- Pengas G, Pereira JM, Williams GB, Nestor PJ. Comparative reliability of total intracranial volume estimation methods and the influence of atrophy in a longitudinal semantic dementia cohort. J Neuroimaging. 2009;19(1):37–46. doi: 10.1111/j.1552-6569.2008.00246.x. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Hyder F, Blamire AM, Shulman RG. FMRI of the prefrontal cortex during overt verbal fluency. NeuroReport. 1997;8(2):561–565. doi: 10.1097/00001756-199701200-00036. [DOI] [PubMed] [Google Scholar]
- Portocarrero JS, Burright RG, Donovick PJ. Vocabulary and verbal fluency of bilingual and monolingual college students. Arch Clin Neuropsychol. 2007;22(3):415–422. doi: 10.1016/j.acn.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Prata DP, Mechelli A, Fu CH, Picchioni M, Kane F, Kalidindi S, McDonald C, Howes O, Kravariti E, Demjaha A, Toulopoulou T, Diforti M, Murray RM, Collier DA, McGuire PK. Opposite effects of catechol-o-methyltransferase Val158Met on cortical function in healthy subjects and patients with schizophrenia. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: contributions from functional neuroimaging. J Anat. 2000;197(Pt 3):335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Moelter ST, Bhati MT, Valdez JN, Kohler CG, Siegel SJ, Gur RC, Gur RE. Effect of retrieval effort and switching demand on fMRI activation during semantic word generation in schizophrenia. Schizophr Res. 2008;99(1–3):312–323. doi: 10.1016/j.schres.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EM, McGovern D, Mockler D, Doku VC, OCS, Fannon DG, Tennakoon L, Santamaria M, Soni W, Morris RG, Sharma T. Neuropsychological functioning in first-episode psychosis—evidence of specific deficits. Schizophr Res. 2000;43(1):47–55. doi: 10.1016/s0920-9964(99)00177-2. [DOI] [PubMed] [Google Scholar]
- Schaufelberger M, Senhorini MC, Barreiros MA, Amaro E, Jr, Menezes PR, Scazufca M, Castro CC, Ayres AM, Murray RM, McGuire PK, Busatto GF. Frontal and anterior cingulate activation during overt verbal fluency in patients with first episode psychosis. Rev Bras Psiquiatr. 2005;27(3):228–332. doi: 10.1590/s1516-44462005000300013. [DOI] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22(3):1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Seldon HL. Does brain white matter growth expand the cortex like a balloon? Hypothesis and consequences. Laterality. 2005;10(1):81–95. doi: 10.1080/13576500342000310. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Mrzljak J, Kleinman JE, Herman MM, Goldman-Rakic PS. Regional specificity in the neuropathologic substrates of schizophrenia: a morphometric analysis of Broca’s area 44 and area 9. Arch Gen Psychiatry. 2003;60(1):69–77. doi: 10.1001/archpsyc.60.1.69. [DOI] [PubMed] [Google Scholar]
- Shalom DB, Poeppel D. Functional anatomic models of language: assembling the pieces. Neuroscientist. 2008;14(1):119–127. doi: 10.1177/1073858407305726. [DOI] [PubMed] [Google Scholar]
- Shapleske J, Rossell SL, Simmons A, David AS, Woodruff PW. Are auditory hallucinations the consequence of abnormal cerebral lateralization? A morphometric MRI study of the sylvian fissure and planum temporale. Biol Psychiatry. 2001;49(8):685–693. doi: 10.1016/s0006-3223(00)01006-4. [DOI] [PubMed] [Google Scholar]
- Sharma T, Lancaster E, Sigmundsson T, Lewis S, Takei N, Gurling H, Barta P, Pearlson G, Murray R. Lack of normal pattern of cerebral asymmetry in familial schizophrenic patients and their relatives—The Maudsley Family Study. Schizophr Res. 1999;40(2):111–120. doi: 10.1016/s0920-9964(99)00143-7. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49(1–2):1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AE, Cattapan-Ludewig K, Zmilacher S, Arbach D, Gruber K, Dvorsky DN, Roth B, Isler E, Zimmer A, Umbricht D. Cognitive functioning in the schizophrenia prodrome. Schizophr Bull. 2007;33(3):761–771. doi: 10.1093/schbul/sbm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer IE, Ramsey NF, Kahn RS. Language lateralization in schizophrenia, an fMRI study. Schizophr Res. 2001;52(1–2):57–67. doi: 10.1016/s0920-9964(00)00180-8. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Ramsey NF, Mandl RC, Kahn RS. Language lateralization in monozygotic twin pairs concordant and discordant for handedness. Brain. 2002;125(Pt 12):2710–2718. doi: 10.1093/brain/awf284. [DOI] [PubMed] [Google Scholar]
- Spalletta G, Tomaiuolo F, Marino V, Bonaviri G, Trequattrini A, Caltagirone C. Chronic schizophrenia as a brain misconnection syndrome: a white matter voxel-based morphometry study. Schizophr Res. 2003;64(1):15–23. doi: 10.1016/s0920-9964(03)00010-0. [DOI] [PubMed] [Google Scholar]
- Spaniel F, Tintera J, Hajek T, Horacek J, Dezortova M, Hajek M, Dockery C, Kozeny J, Hoschl C. Language lateralization in monozygotic twins discordant and concordant for schizophrenia. A functional MRI pilot study. Eur Psychiatry. 2007;22(5):319–322. doi: 10.1016/j.eurpsy.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Tae WS, Kim SS, Lee KU, Nam EC, Kim KW. Validation of hippocampal volumes measured using a manual method and two automated methods (FreeSurfer and IBASPM) in chronic major depressive disorder. Neuroradiology. 2008;50(7):569–581. doi: 10.1007/s00234-008-0383-9. [DOI] [PubMed] [Google Scholar]
- Takizawa R, Kasai K, Kawakubo Y, Marumo K, Kawasaki S, Yamasue H, Fukuda M. Reduced frontopolar activation during verbal fluency task in schizophrenia: a multi-channel near-infrared spectroscopy study. Schizophr Res. 2008;99(1–3):250–262. doi: 10.1016/j.schres.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Thorup A, Petersen L, Jeppesen P, Nordentoft M. Frequency and predictive values of first rank symptoms at baseline among 362 young adult patients with first-episode schizophrenia. Results from the Danish OPUS study. Schizophr Res. 2007;97(1–3):60–67. doi: 10.1016/j.schres.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Venkatasubramanian G, Jayakumar PN, Gangadhar BN, Keshavan MS. Automated MRI parcellation study of regional volume and thickness of prefrontal cortex (PFC) in antipsychotic-naive schizophrenia. Acta Psychiatr Scand. 2008;117(6):420–431. doi: 10.1111/j.1600-0447.2008.01198.x. [DOI] [PubMed] [Google Scholar]
- Voets NL, Adcock JE, Flitney DE, Behrens TE, Hart Y, Stacey R, Carpenter K, Matthews PM. Distinct right frontal lobe activation in language processing following left hemisphere injury. Brain. 2006;129(Pt 3):754–766. doi: 10.1093/brain/awh679. [DOI] [PubMed] [Google Scholar]
- Voets NL, Hough MG, Douaud G, Matthews PM, James A, Winmill L, Webster P, Smith S. Evidence for abnormalities of cortical development in adolescent-onset schizophrenia. Neuroimage. 2008;43(4):665–675. doi: 10.1016/j.neuroimage.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Warrier C, Wong P, Penhune V, Zatorre R, Parrish T, Abrams D, Kraus N. Relating structure to function: Heschl’s gyrus and acoustic processing. J Neurosci. 2009;29(1):61–69. doi: 10.1523/JNEUROSCI.3489-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisco JJ, Kuperberg G, Manoach D, Quinn BT, Busa E, Fischl B, Heckers S, Sorensen AG. Abnormal cortical folding patterns within Broca’s area in schizophrenia: evidence from structural MRI. Schizophr Res. 2007;94(1–3):317–327. doi: 10.1016/j.schres.2007.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki S, Yamasue H, Abe O, Yamada H, Iwanami A, Hirayasu Y, Nakamura M, Furukawa S, Rogers MA, Tanno Y, Aoki S, Kato N, Kasai K. Reduced planum temporale volume and delusional behaviour in patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2007;257(6):318–324. doi: 10.1007/s00406-007-0723-5. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Yamada H, Yumoto M, Kamio S, Kudo N, Uetsuki M, Abe O, Fukuda R, Aoki S, Ohtomo K, Iwanami A, Kato N, Kasai K. Abnormal association between reduced magnetic mismatch field to speech sounds and smaller left planum temporale volume in schizophrenia. Neuroimage. 2004;22(2):720–727. doi: 10.1016/j.neuroimage.2004.01.042. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Shi J, Yuan Y, Hao G, Yao Z, Chen N. Relationship of auditory verbal hallucinations with cerebral asymmetry in patients with schizophrenia: an event-related fMRI study. J Psychiatr Res. 2008;42(6):477–486. doi: 10.1016/j.jpsychires.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Suzuki M, Takahashi T, Hagino H, Kawasaki Y, Matsui M, Seto H, Kurachi M. Parietal lobe volume deficits in schizophrenia spectrum disorders. Schizophr Res. 2007;89(1–3):35–48. doi: 10.1016/j.schres.2006.08.032. [DOI] [PubMed] [Google Scholar]