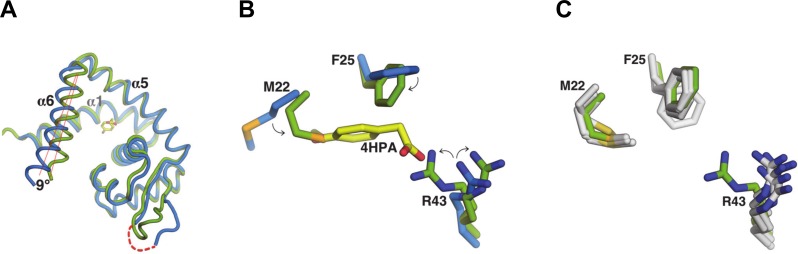
Fig 5. Structural differences of NadR in ligand-bound or free forms.
(A) Aligned monomers of holo-NadR (chain A: green; chain B: blue), reveal major overall differences by the shift of helix α6. (B) Comparison of the two binding pockets in holo-NadR shows that in the ligand-free monomer A (green) residues Met22, Phe25 and Arg43 adopt ‘inward’ positions (highlighted by arrows) compared to the ligand-occupied pocket (blue residues); these ‘inward’ conformations appear unfavorable for binding of 4-HPA due to clashes with the 4-hydroxyl group, the phenyl ring and the carboxylate group, respectively. In these crystals, the ArgA43 side chain showed two alternate conformations, modelled with 50% occupancy in each state, as indicated by the two ‘mirrored’ arrows. The inner conformer is the one that would display major clashes if 4-HPA were present. (C) Comparison of the empty pocket from holo-NadR (green residues) with the four empty pockets of apo-NadR (grey residues), shows that in the absence of 4-HPA the Arg43 side chain is always observed in the ‘outward’ conformation.