Figure 2.
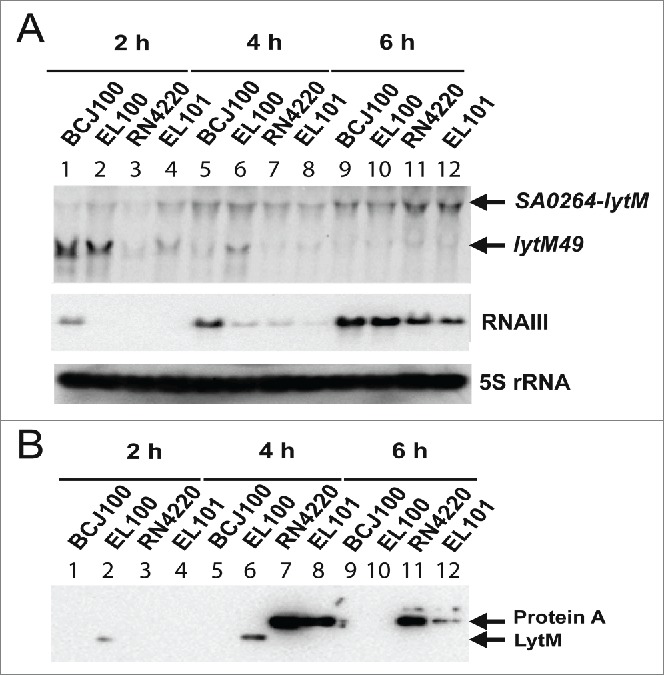
Analysis of LytM synthesis in various strain backgrounds. (A) Northern blot experiments showing the steady state levels of lytM mRNAs (top), of RNAIII (middle) and of 5S RNA (loading control, bottom). Total RNAs were prepared after 2 h (lanes 1 to 4), 4 h (lanes 5 to 8) and 6 h (lanes 9 to 12) of culture in BHI at 37°C from 2 strain backgrounds, BCJ100 (HG001, ΔhsdR Δtype III) and RN4220. A FLAG tag was introduced at the 3′ end of lytM gene in BCJ100 (EL100) and in RN4220 (EL101) to analyze the synthesis of LytM in the 2 strain backgrounds. Experiments were performed on samples prepared from BCJ100 (lanes 1, 5, 9), EL100 (lanes 2, 6, 10), RN4220 (lanes 3, 7, 11) and EL101 (lanes 4, 8, 12). lytM49, corresponds to the WalKR-induced lytM mRNA. (B) Western blot performed using monoclonal antibodies against FLAG tag on protein extracts prepared from the same strains as described for the Northern experiment (see also Material and Methods). For the 2 h samples, the equivalent of 0.8 OD units was loaded, while for the 4 and 6 h samples, the equivalent of 1 OD unit was loaded in order to visualize LytM. As a control, a gel run in parallel with the same samples was stained with Coomassie blue to verify that each lane contained comparable amounts of protein (not shown). The legend is the same as for Fig. 2A. Lanes 7–8 and 11–12: protein A reacted with the anti-Flag antibody in RN4220 and EL101 because RNAIII is weakly expressed. RNAIII is known to repress the translation of spa mRNA encoding protein A through basepairing interactions.34
