Figure 4.
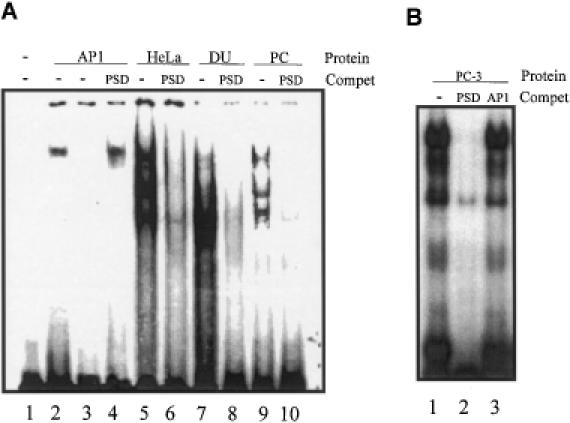
Gel shift analyses of nuclear proteins binding to the PSD oligonucleotide. 32P-labeled oligonucleotide probes were incubated with nuclear extract proteins from HeLa, DU 145 or PC-3 cells. Protein–DNA complexes were resolved on 4% non-denaturing polyacrylamide gel and visualized using autoradiography. (A) Lane 1, PSD probe alone; lane 2, end-labeled AP1 consensus oligonucleotide incubated with AP1 protein; lane 3, end-labeled PSD consensus oligonucleotide incubated with AP1 protein; lane 4, end-labeled AP1 consensus oligonucleotide incubated with AP1 protein in the presence of 100-fold molar excess of unlabeled double-stranded PSD oligonucleotide; lanes 5–10, the PSD nucleotide was end-labeled and incubated with HeLa, DU 145 or PC-3 nuclear extracts in the absence or presence of 100-fold molar excess of unlabeled double-stranded PSD; lanes 5 and 6, HeLa extract; lanes 7 and 8, DU 145 extract; lanes 9 and 10, PC-3 extract. Reactions were performed with ∼0.03 ng of radiolabeled probe (30 000 c.p.m.) and 5 μg nuclear extracts. (B) Lane 1, the PSD probe alone was incubated with 10 μg of PC-3 nuclear extracts; lanes 2 and 3, PSD nucleotide was incubated with 10 μg of PC-3 cell nuclear extract proteins with a 100-fold molar excess of unlabeled PSD or AP1 consensus oligonucleotides.
