Figure 2.
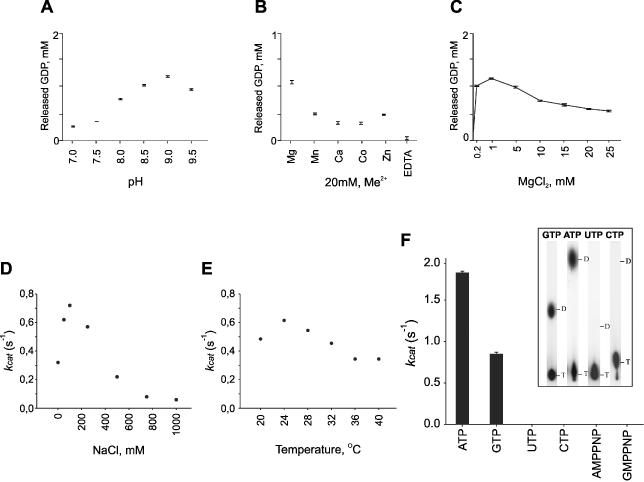
Effects of the reaction conditions on the activity of the φ12 P4 NTPase. (A–C) Quantitative analysis by TLC showing the effects of pH [(A) 20 mM Tris–HCL, 50 mM NaCl, 5 mM MgCl2], divalent metals—Me2+ [(B) 20 mM Tris–HCl, pH 8.0, 50 mM NaCl, 0.2 mM MgCl2, 20 mM Me2+] and Mg2+ concentration [(C) 20 mM Tris–HCl, pH 8.0, 50 mM NaCl], on the GTPase activity of φ12 P4 (GTP concentration was 2 mM, protein concentration 0.25 mg/ml) at 37°C. (D–F) P4 NTP turnover (kcat) was measured using steady state kinetics of Pi release (NTP concentration 1 mM) under different conditions: NaCl effect [(D) 20 mM Tris–HCl, pH 9.0, 1 mM MgCl2, 24°C)], temperature dependence [(E) 20 mM Tris–HCl, pH 9.0, 1 mM MgCl2, 100 mM NaCl)], hydrolysis of different nucleotides [(F) NTPs, AMPPNP and GMPPNP concentration 1 mM, 20 mM Tris–HCl, pH 9.0, 1 mM MgCl2, 100 mM NaCl, 24°C], and TLC run showing the hydrolysis of different nucleotides under optimal conditions (insert). The analyses were repeated three times and error bars show standard deviations.
