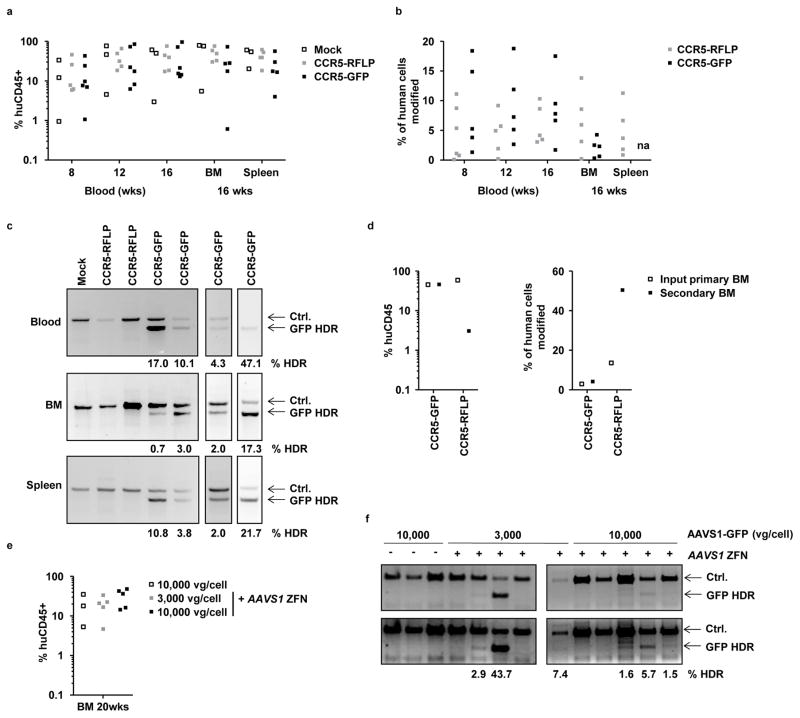
Figure 6. Engraftment of NSG mice with gene edited HSPCs.
(a) Neonatal NSG mice were engrafted with fetal liver HSPCs, either mock treated, or treated with AAV6 donors (CCR5-GFP or CCR5-RFLP) plus CCR5 ZFN mRNAs, using 2 different donor tissues. Genome editing levels in these input HSPCs were 9.1% and 12% for CCR5-GFP treated cells, by flow cytometry, and 6.7% and 11.2% for CCR5-RFLP treated cells, by deep sequencing. Peripheral blood of the mice was analyzed at weeks 8, 12, and 16 for the % of human CD45+ cells, and bone marrow (BM) and spleen were analyzed at 16 weeks. Shown is the combined data from the two separate cohorts of mice. No significant differences were found in the levels of human cells in the blood or tissues between mock and treated samples (two-way ANOVA). (b) Rates of genome modification in human cells were measured in blood and tissue samples from individual mice by flow cytometry for GFP insertions, or deep sequencing for RFLP insertions; na, not available due to high background autofluorescence of cells. Actual numbers are available in Supplemental Table 3. Cells from mock-treated mice gave only background levels in all assays (not shown). (c) Representative examples of In-Out PCR showing GFP addition at the CCR5 locus in peripheral blood, bone marrow, and spleen from individual mice at 16 weeks post engraftment. Numbers for any samples above the levels of the background controls are shown. (d) Bone marrow was isolated at 16 weeks post-engraftment from 2 mice each from the CCR5-GFP or CCR5-RFLP cohorts and was pooled. The levels of human CD45+ cell engraftment and gene modification (GFP+ by flow cytometry, RFLP insertion by deep sequencing) were measured in the pooled cell populations. Each primary BM pool was used to transplant a separate adult female NSG mouse and, 20 weeks later, bone marrow was isolated from the secondary transplant recipients and analyzed for human CD45+ content and levels of genome modification in the same way. (e) Mobilized blood HSPCs were treated with AAVS1-GFP vectors, with and without AAVS1 ZFN, and used to engraft NSG mice. The frequency of GFP+ cells in the input HSPCs, measured at 5 days post-transfection in culture, were 0.72%, 20.5% and 30.7% respectively, for cells receiving 10,000 vg/cell AAVS1-GFP alone, 3,000 vg/cell AAVS1-GFP plus ZFNs and 10,000 vg/cell AAVS1-GFP plus ZFNs. At 20 weeks, bone marrow was isolated and analyzed for human CD45+ leucocytes. (f) Detection of GFP insertion at the AAVS1 locus in bone marrow samples from individual mice, measured by In-Out PCR. Numbers for any samples above the levels of the background controls are shown. Uncropped images of all gels in this figure are available in Supplemental Figure 13.