Abstract
Objectives
To evaluate the feasibility and repeatability of applying blood oxygen level-dependent (BOLD) magnetic resonance (MR) imaging in the feet to quantify regional dynamic changes in tissue oxygenation during proximal cuff occlusion and reactive hyperemia.
Subjects and Methods
Ten healthy male subjects underwent BOLD and T1-weighted imaging of the feet on two separate occasions using a 3T scanner. Dynamic changes in BOLD signal intensity were assessed before and during proximal cuff occlusion of the thigh and during reactive hyperemia, and BOLD time course data was evaluated for the time-to-half ischemic minimum, minimum ischemic value, peak hyperemic value, time-to-peak hyperemia, time-to-half peak hyperemia, and end value. T1-weighted images were used for segmentation of volumes of interest (VOI) in anatomical regions of the foot (heel, toes, dorsal foot, medial and lateral plantar foot). Repeatability of vascular responses was assessed for each foot VOI using semi-automated image registration and quantification of serial BOLD images.
Results
The heel VOI demonstrated a significantly higher peak hyperemic response, expressed as percent change from baseline BOLD signal intensity, compared to all other VOIs of the foot (heel, 7.4±1.2%; toes, 5.6±0.8%; dorsal foot, 5.7±1.6%; medial plantar, 5.6±1.7%; lateral plantar, 5.6±1.5%; P<0.05). Additionally, the lateral plantar VOI had a significantly lower terminal signal intensity value (i.e., end value) when compared to all foot VOIs (P<0.05). BOLD MR imaging was repeatable between visits in all foot VOIs, with no significant differences between study visits for any of the evaluated functional indices.
Conclusions
BOLD MR imaging offers a repeatable technique for volumetric assessment of regional foot tissue oxygenation. Future application of BOLD imaging in the feet of patients with peripheral vascular disease may permit serial evaluation of regional tissue oxygenation and allow for improved assessment of therapeutic interventions targeting specific sites of the foot.
Keywords: Magnetic resonance imaging, reactive hyperemia, skeletal muscle, diabetes, peripheral vascular disease
INTRODUCTION
Blood oxygen level-dependent (BOLD) magnetic resonance (MR) is a non-invasive technique originally developed for functional brain imaging that has gained attention for evaluation of dynamic changes in skeletal muscle tissue oxygenation.1 The BOLD signal is sensitive to relative changes in paramagnetic deoxyhemoglobin and diamagnetic oxyhemoglobin content within the microvasculature. As the concentration of paramagnetic deoxyhemoglobin increases, signal intensity decreases on T2-weighted BOLD images, which is attributed to an increase in intravoxel spin dephasing. Because BOLD signal is based on the status of intravascular hemoglobin oxygenation, shifts in BOLD signal intensity may be dependent on several factors, including muscle blood volume, perfusion, fluid shifts, metabolic factors, vascular architecture, and magnetic field angulation; however, dynamic changes in BOLD signal are thought to primarily reflect fluctuations in bulk blood flow, particularly within the microvasculature.1
Previous studies have quantified regional alterations in tissue oxygenation within calf muscles by using reactive hyperemia,2–4 exercise,5 and oxygen ventilation 6 paradigms to induce changes in blood flow and thus BOLD signal intensity. Kos et al. 7 examined dynamic changes in BOLD signal within a single slice of the mid-foot during transient ischemia and reactive hyperemia, demonstrating that BOLD may also have potential clinical application for evaluating microvascular perfusion and tissue oxygenation within the feet. The ability to detect microvascular foot abnormalities may be particularly valuable in the setting of diabetes, where patients commonly present with concomitant macrovascular and microvascular disease. Although various techniques, such as transcutaneous oxygen pressure and laser Doppler flowmetry, are available to assess the adequacy of skin blood flow, these approaches have not been readily adopted due to lengthy acquisition times and reproducibility concerns.8,9 Additionally, these tools do not permit quantitative volumetric assessment of tissue oxygenation or perfusion within different territories of the foot. Therefore, a clinical need exists for non-invasive tools capable of assessing global changes in foot tissue oxygenation and perfusion following treatment. Further development of BOLD imaging for assessment of regional, volumetric tissue oxygenation may provide a more robust technique for evaluating the foot, which is the most distal vascular territory of the lower extremity and is therefore highly susceptible to decreased tissue oxygenation in the setting of arterial stenosis or occlusion.
Our research team has previously developed image segmentation tools that have been applied for serial analysis of lower extremity tissue perfusion 10 and angiogenesis.11 In the present study, we sought to expand on these tools by developing a segmental three-dimensional (3D) model of the foot to quantify volumetric tissue oxygenation within specific territories. Our group has also previously developed tools for serial image registration 12 that we further apply to T1-weighted and BOLD images for improved serial quantitative analysis of BOLD signal within the foot.
Therefore, the primary goals of this study were to evaluate the feasibility of quantifying volumetric tissue oxygenation in a segmental model of the foot using BOLD MR imaging and to test the repeatability of BOLD imaging during transient ischemia and reactive hyperemia in healthy volunteers. Future application of BOLD imaging in the feet of patients with peripheral vascular disease (PVD) and/or diabetes may permit serial evaluation of regional tissue oxygenation and allow for improved assessment of responses to therapeutic interventions.
SUBJECTS AND METHODS
Research Subjects
Ten healthy male subjects participated in the MR research protocol. A standard medical history questionnaire was administered to evaluate each subject's risk factors for cardiovascular, pulmonary, and metabolic disease, which included history of smoking, hypertension, diabetes, hypercholesterolemia, and family history of coronary artery disease. Due to the potential impact of physical activity on vascular function,13 the International Physical Activity Questionnaire (IPAQ) was also administered to evaluate each subject's activity levels for the week prior to study involvement. The study was approved by the Institutional Review Board for Human Subjects Research and Review Committee and was in accordance with the guidelines set forth by the Declaration of Helsinki. Subjects reported for imaging following an 8-hour fasting period that also consisted of abstinence from caffeine and alcohol. All individuals provided written informed consent after receiving an explanation of the experimental procedures and potential risks associated with participating in the study.
MR Imaging Protocol
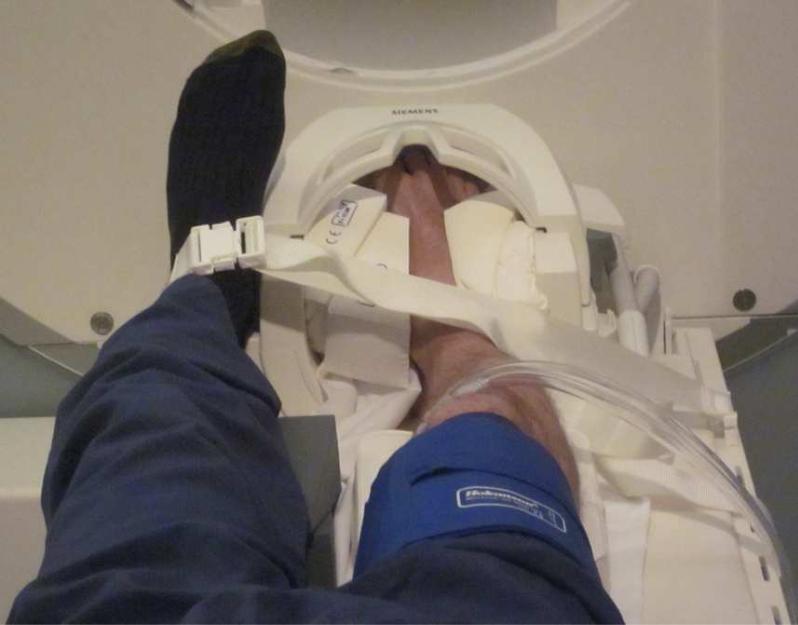
Subjects reported for MR imaging on two separate occasions with each visit being at least one week apart. Resting blood pressure and heart rate measurements were manually acquired prior to the start of each imaging session to ensure consistent hemodynamics. All subjects underwent BOLD and T1-weighted FLASH scanning of the foot on a 3 Tesla MR system (TIM Trio; Siemens Healthcare, Erlangen, Germany). Each subject's right foot was placed up to the level of the ankle inside a 32-channel phased array head coil (Siemens) and firmly secured to restrict movement (Figure 1). A blood pressure cuff was secured around the thigh for rapid inflation (ischemia) and deflation (reactive hyperemia) using a Hokanson E20 AG101 Rapid Cuff Inflation System (D.E. Hokanson, Inc; Bellevue, WA). One BOLD scan was performed during each visit and consisted of 930 sequential total volumes. The BOLD scan lasted 15 min, with the first 5 min representing the baseline assessment, followed by a 5 min occlusion phase (cuff pressure inflated to 50 mmHg above resting systolic pressure) and 5 min of reactive hyperemia following cuff deflation. Multi-band BOLD 14 imaging parameters were as follows: FOV = 280×140 mm2; matrix = 128×64; number of slices = 51 (axial); slice thickness = 2.2 mm (no gap); TR/TE = 960/30 ms; flip angle = 80 degrees; multi-band acceleration factor = 3, and parallel imaging factor = 2. Following each BOLD acquisition, T1-weighted imaging was performed, with slices matched to BOLD images using the same FOV to aid in foot segmentation and co-registration of BOLD and T1 images between study visits. T1 imaging parameters were as follows: in-plane matrix = 192×96; TR/TE = 285/2.61 ms; flip angle = 70 degrees; and 3 averages for noise suppression.
Figure 1.
Experimental set-up for creating transient ischemia and reactive hyperemia. The foot was firmly secured within a phased array head coil with blood pressure cuff positioned over the mid-thigh. Contralateral foot was supported and secured alongside head coil.
Image Registration and Segmentation
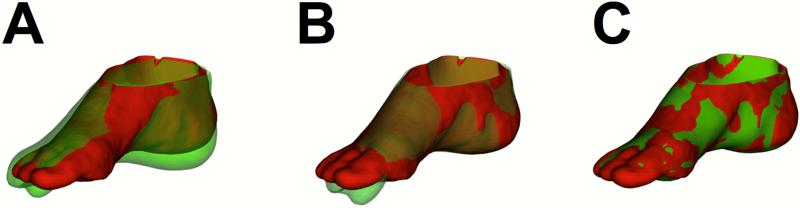
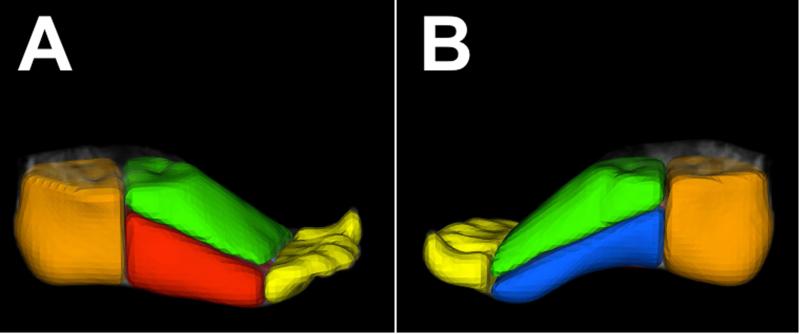
Figure 2 provides an illustration of the adaptation of our two-stage method of image registration, which involves initial global rigid alignment followed by non-rigid registration.12 Volumes of interest (VOIs) were generated in the foot from co-registered T1-weighted images using a previously validated image segmentation tool 11 by drawing planes through the foot using specific bones as reference points to standardize the segmentation process (Figure 3). Briefly, the heel VOI was defined by drawing a plane in front of the most distal point of the calcaneus bone. The toes were segmented by using the most distal point of the metatarsals as a cutoff point. VOIs for the dorsal and plantar halves of the foot were generated by drawing a plane that began at the point between calcaneus and talus and continued to the toes by cutting through the middle of the metatarsals. The plantar foot was further divided by drawing a plane that ran directly through the second metatarsal. All image registration and segmentation was performed using BioImage Suite (http://www.bioimagesuite.org), an image analysis toolkit.15
Figure 2.
Serial image registration of the foot. Registration was performed using points sampled from outer skin surfaces. Shown are the rendered surfaces from T1-weighted MR images from two separate study visits (red=visit 1; green=visit 2) at the (A) starting position before registration, (B) after global rigid alignment, and (C) after non-rigid registration.
Figure 3.
Foot volumes of interest (VOIs). Lateral (A) and medial (B) views of a three-dimensional representation of regional foot segmentation generated from T1-weighted MR images. VOIs for the heel (orange), dorsal foot (green), lateral plantar (red), medial plantar (blue), and toes (yellow) are displayed.
Image Analysis
Time course data was generated to evaluate dynamic changes in BOLD signal intensity for each foot VOI. Image intensity values were expressed as a percent change from resting baseline signal intensity (i.e., image intensity value – baseline SI ÷ baseline SI). Six parameters were evaluated during transient ischemia and reactive hyperemia. Parameters included: 1) time-to-half ischemic minimum; 2) minimum ischemic value; 3) peak hyperemic value, defined as the highest five-second average value recorded following cuff deflation; 4) time to peak, which refers to the time from cuff deflation to peak BOLD signal; 5) time-to-half peak; and 6) end value, which was the 10 second average value during the final seconds of the BOLD acquisition. Repeatability for each of the six parameters was evaluated within each of the five foot VOIs.
Statistical Analyses
Analysis of variance (ANOVA) was used to identify differences in BOLD signal intensity responses between each foot VOI. Paired analysis was performed for evaluating differences in BOLD parameters between study visits (time). All statistical analyses were performed using commercially available software (GraphPad Prism v6.0 for Mac OS X, GraphPad Software, La Jolla, CA, USA). Statistical significance for all analyses was set at P<0.05. All values are expressed as means ± SD unless stated otherwise.
RESULTS
Research Subjects
The subject demographics are summarized in Table 1. Medical history questionnaires confirmed that all individuals were void of cardiovascular, pulmonary, and metabolic disease, and did not have significant risk factors for cardiovascular disease. The IPAQ identified low levels of physical activity in subjects the week prior to study involvement (Table 1), which was maintained throughout the duration of the study.
Table 1.
Subject Characteristics
| Age, yrs | 24.6 ± 3.0 |
| Height, cm | 179.1 ± 5.1 |
| Body mass, kg | 80.7 ± 17.6 |
| Systolic blood pressure, mmHg | 119.9 ± 14.0 |
| Diastolic BP, mmHg | 71.2 ± 11.7 |
| Resting heart rate, bpm | 76.5 ± 9.8 |
| Walking, hrs/week | 7.4 ± 10.0 |
| Moderate exercise, hrs/week | 1.1 ± 2.1 |
| Vigorous exercise, hrs/ week | 0.3 ± 0.7 |
| Sitting, hrs/week | 67.6 ± 32.6 |
Analysis of BOLD Imaging
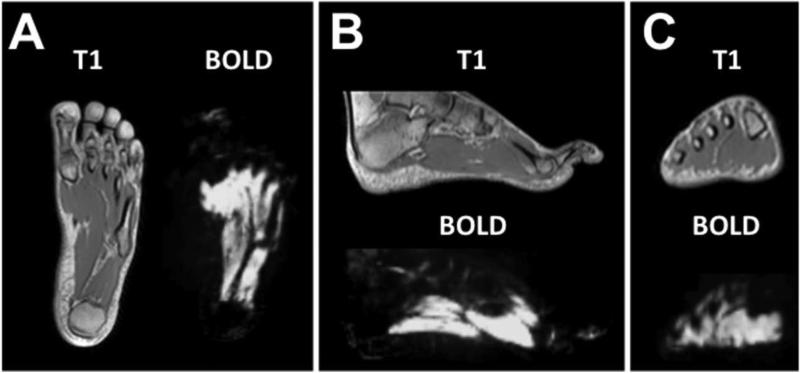
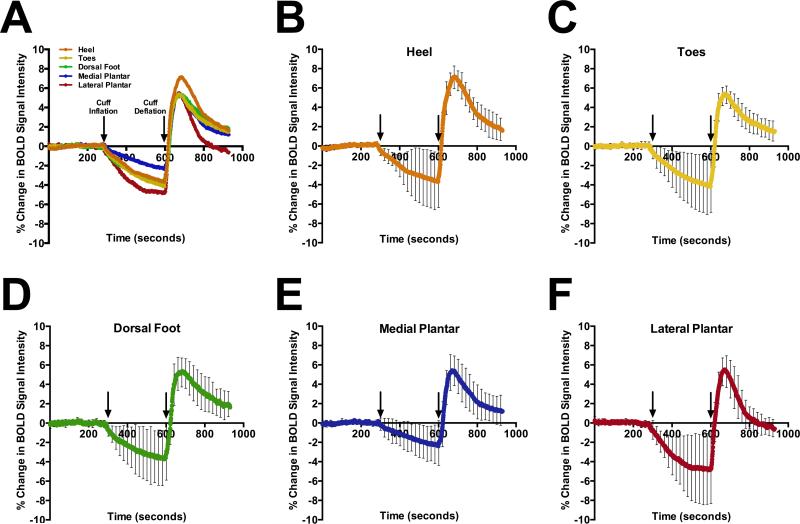
BOLD imaging of the foot produced a quantifiable signal, which was localized to soft tissue within vascular territories of the foot using co-registered T1-weighted anatomical images (Figure 4). The average time dependent changes and differences in the dynamic BOLD signal between individual foot VOIs during baseline, cuff occlusion, and reactive hyperemia are shown in Figure 5A. Individual graphs of dynamic changes in BOLD signal for each foot VOIs are also provided (Figure 5 B-F) with associated standard deviations, illustrating the time dependent variability in BOLD signal among the subjects for each foot VOI. Regional differences in the average quantitative BOLD indices of the foot during cuff occlusion and reactive hyperemia are summarized in Table 2 for all 10 subjects. Peak hyperemic value was significantly higher in the heel compared to all other VOIs (P<0.05). The end BOLD value following 5 minutes of reactive hyperemia was significantly lower in the lateral plantar VOI when compared to all other foot VOIs (P<0.05). Additionally, the medial plantar VOI was significantly different from the lateral plantar VOI in the assessment of minimum ischemic value (P=0.01). No other significant differences were observed between foot VOIs when evaluating regional BOLD indices.
Figure 4.
A) Axial, (B) sagittal, and (C) coronal views of T1-weighted and BOLD MR images of the foot.
Figure 5.
A) Average BOLD time course curves for each foot VOI demonstrate regional variability during occlusion and reactive hyperemia while individual response curves for the (B) heel, (C) toes, (D) dorsal foot, (E) medial plantar, and (F) lateral plantar VOI demonstrate inter subject variability. Arrows indicate time of cuff inflation (initialization of ischemia) and deflation (start of reactive hyperemia phase). Values represent average ± SD. N=10 subjects.
Table 2.
BOLD Responses
| FOOT VOLUME OF INTEREST | |||||
|---|---|---|---|---|---|
| Heel | Toes | Dorsal foot | Medial plantar | Lateral plantar | |
| Time-to-half ischemia, sec | 54.8±35.3 | 64.3±42.2 | 60.5±49.3 | 70.6±54.3 | 48.5±24.6 |
| Minimum ischemic value, % | −4.1±3.0 | −4.5±3.2 | −4.2±2.7 | −2.8±1.7† | −5.1±2.8 |
| Time-to-half peak, sec | 32.5±13.7 | 32.8±10.7 | 34.5±6.2 | 33.2±12.6 | 34.3±11.7 |
| Time-to-peak, sec | 75.9±17.3 | 78.4±25.5 | 80.1±19.0 | 70.9±11.3 | 65.5±13.3 |
| Peak hyperemic value, % | 7.4±1.2* | 5.6±0.8 | 5.7±1.6 | 5.6±1.7 | 5.6±1.5 |
| End value, % | 1.7±1.2 | 1.5±1.0 | 1.8±1.2 | 1.2±1.5 | −0.6±0.9* |
Values are means ± SD.
significantly different from all other VOIs.
significantly different from lateral plantar VOI, P < 0.05. N=10 subjects.
Test-Retest Repeatability
Baseline hemodynamic measurements between the two study visits were not different for systolic (visit 1, 119±14.0 mmHg; visit 2, 120.4±11.0 mmHg; p=0.7) or diastolic (71.2±11.7 mmHg vs. 72.4±8.2 mmHg; p=0.4) blood pressure, and resting heart rate was similar (76.5±9.8 bpm vs. 74.7±7.7 bpm; p=0.2). Table 2 provides a summary of the quantitative index from each VOI for the dynamic BOLD images obtained during occlusion and reactive hyperemia. All BOLD indices were repeatable for each VOI, with no significant differences observed between study visits for time to half ischemia, minimum ischemic value, time to half peak, time to peak, peak hyperemic value, and end value.
DISCUSSION
In the present study, we demonstrate the feasibility of quantifying regional dynamic changes in foot tissue oxygenation using BOLD MR imaging during proximal cuff occlusion and reactive hyperemia. We also demonstrate that BOLD indices of regional tissue oxygenation were repeatable between study visits. This repeatability was in part due to our semi-automated approach for registration of the three-dimensional images and segmentation of the foot into specific territories using anatomical T1-weighted images. All VOIs of the foot demonstrated robust dynamic changes in BOLD signal intensity that were quantified during phases of tissue ischemia and hyperemia. Significantly higher peak hyperemic values were found in the heel VOI of the foot compared to all other segmented VOIs, while significantly lower end values were observed in the lateral plantar VOI when compared to other VOIs.
Previous studies have evaluated dynamic changes in calf tissue oxygenation using BOLD imaging;3,2,4 however, to our knowledge, only one previous study has applied BOLD imaging to investigate foot tissue oxygenation.7 In this previous work by Kos et al.,7 which incorporated a reactive hyperemia paradigm, the authors assumed that all muscle tissue within the foot would have homogeneous vascular responses based on similarities in tissue composition. Because of this assumption, Kos et al. only examined dynamic changes in BOLD signal intensity within a single slice of the plantar muscles in the foot. In the present study, however, we developed and applied a segmental 3D model of the foot to evaluate dynamic changes in BOLD signal within multiple VOIs of the foot and demonstrate regional heterogeneity in response to reactive hyperemia. Specifically, we observed significant regional variation in the peak hyperemic value and the BOLD value at the end of reactive hyperemia. These regional differences in vascular responses may be attributed to differences in vascular supply, as the foot and ankle can be divided into various angiosomes, or 3D vascular territories, where each angiosome is supplied by branches of the anterior tibial, posterior tibial, and peroneal artery.16 The higher peak hyperemic response observed in the heel VOI may be attributed to the fact that the plantar heel is supplied by calcaneal branches from both the posterior tibial and peroneal arteries, providing the heel with potentially higher arterial blood supply during reactive hyperemia. By comparison, the lateral plantar VOI, which demonstrated the lowest end value compared to other VOIs, is only supplied by the lateral plantar branch of the posterior tibial artery. Although only speculative, the significant difference observed in the BOLD value at the end of hyperemia within the lateral plantar VOI may be attributed to a more efficient venous network that allows for a faster departure of blood volume and an increased return to baseline BOLD signal intensity following reactive hyperemia. From the current study we cannot determine if the observed regional differences in tissue oxygenation during occlusion and reactive hyperemia are due to regional variations in larger arterial supply, or the regional microvasculature. This issue could be potentially solved by acquisition of additional MR imaging sequences such as arterial spin labeling, an approach that provides an index of tissue perfusion, or time-of-flight imaging, which characterizes the vascular network.
In addition to regional analysis of dynamic changes in foot tissue oxygenation, we applied a previously developed tool for serial image registration to test the repeatability of the quantitative BOLD measurements within each VOI of the foot.12 Using this technique for serial comparisons, we found that all BOLD imaging parameters examined were repeatable between study visits for each VOI of the foot.
Different levels of inter-subject variability were observed in all VOIs of the foot during the BOLD imaging protocol, with this variability being particularly pronounced during ischemia-induced cuff occlusion of the leg (Figure 5 B-F). Contrary to the inter-subject variability observed during the cuff occlusion phase, BOLD signal responses appear to be less variable during the reactive hyperemia phase within the present imaging protocol. This finding suggests that future MR imaging studies incorporating reactive hyperemia paradigms in the lower extremities may choose to restrict evaluation of the BOLD signal to the reactive hyperemia phase, where these measures may provide the least amount of variability and potentially produce the most valid approach for assessment of tissue health and viability.
MR angiography is a cost effective tool in the evaluation of patients with PVD and also produces a higher cost-to-quality of life ratio when compared to standard digital subtraction angiography;17 however, alternative imaging modalities such as MR imaging continue to be underutilized and their use remains highly variable based on geographic location and institution.18 Future application of BOLD MR imaging combined with MR angiography may provide an additional non-contrast, cost effective technique that offers complementary information in the assessment of PVD patients.
CONCLUSIONS
The application of noninvasive dynamic BOLD MR imaging with quantification of regional volumetric tissue oxygenation may provide insight into the functional improvements in downstream vascular territories of the foot following therapeutic interventions. Future investigations should focus on evaluating the response to targeted revascularization in patients with diabetes and PVD to fully elucidate the clinical potential of this technique.
WHAT THIS PAPER ADDS
This study is the first to evaluate dynamic changes in tissue oxygenation within three-dimensional regions of the foot using blood oxygen level-dependent (BOLD) magnetic resonance imaging (MRI). Regional differences in foot tissue oxygenation were quantified in response to a cuff occlusion and reactive hyperemia paradigm, and these MRI-derived quantitative indices were repeatable in healthy volunteers. Clinical translation of this work in patients with diabetes and/or peripheral vascular disease may provide novel non-invasive insight into downstream physiological changes that occur within specific regions of the foot following surgical, endovascular, and medical treatment.
ACKNOWLEDGEMENTS
This research was financially supported in part by a grant from the National Football League (NFL) Charities (AJ Sinusas). The views expressed in this work are those of the author/s and do not necessarily reflect the views or policies of the NFL or NFL Charities. This work was also supported in part by NIH grant T32 HL098069 (AJ Sinusas).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
None.
REFERENCES
- 1.Jacobi B, Bongartz G, Partovi S, Schulte A-C, Aschwanden M, Lumsden A, et al. Skeletal muscle BOLD MRI: From underlying physiological concepts to its usefulness in clinical conditions. J Magn Reson Imaging. 2012;35:1253–65. doi: 10.1002/jmri.23536. [DOI] [PubMed] [Google Scholar]
- 2.Ledermann H-P, Schulte A-C, Heidecker H-G, Aschwanden M, Jäger KA, Scheffler K, et al. Blood oxygenation level-dependent magnetic resonance imaging of the skeletal muscle in patients with peripheral arterial occlusive disease. Circulation. 2006;113(25):2929–35. doi: 10.1161/CIRCULATIONAHA.105.605717. [DOI] [PubMed] [Google Scholar]
- 3.Ledermann HP, Heidecker H-G, Schulte A-C, Thalhammer C, Aschwanden M, Jaeger KA, et al. Calf muscles imaged at BOLD MR: correlation with TcPO2 and flowmetry measurements during ischemia and reactive hyperemia-initial experience. Radiology. 2006;241(2):477–84. doi: 10.1148/radiol.2412050701. [DOI] [PubMed] [Google Scholar]
- 4.Utz W, Jordan J, Niendorf T, Stoffels M, Luft FC, Dietz R, et al. Blood oxygen level-dependent MRI of tissue oxygenation. Relation to endothelium-dependent and endothelium-independent blodo flow changes. Arter Thromb Vasc Biol. 2005;25:1408–13. doi: 10.1161/01.ATV.0000170131.13683.d7. [DOI] [PubMed] [Google Scholar]
- 5.Towse TF, Slade JM, Meyer RA. Effect of physical activity on MRI-measured blood oxygen level-dependent transients in skeletal muscle after brief contractions. J Appl Physiol. 2005;99:715–22. doi: 10.1152/japplphysiol.00272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noseworthy MD, Kim JK, Stainsby JA, Stanisz GJ, Wright GA. Tracking oxygen effects on MR signal in blood and skeletal muscle during hyperoxia exposure. J Magn Reson Imaging. 1999;9:814–20. doi: 10.1002/(sici)1522-2586(199906)9:6<814::aid-jmri8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Kos S, Klarhofer M, Aschwanden M, Scheffler K, Jacob AL, Bilecen D. Simultaenous dynamic blood oxygen level-dependent magnetic resonance imaging of foot and calf muscles. Aging effects at ischemia and postoscclusive hyperemia in healthy volunteers. Invest Radiol. 2009;44:741–7. doi: 10.1097/RLI.0b013e3181b248f9. [DOI] [PubMed] [Google Scholar]
- 8.Yip WL. Evaluation of the clinimetrics of transcutaneous oxygen measurement and its application in wound care. Int Wound J. 2015;12(6):625–9. doi: 10.1111/iwj.12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humeau-Heurtier A, Guerreschi E, Abraham P, Mahe G. Relevance of laser Doppler and laser speckle techniques for assessing vascular function: state of the art and future trends. IEEE Trans Biomed Eng. 2013;60(3):659–66. doi: 10.1109/TBME.2013.2243449. [DOI] [PubMed] [Google Scholar]
- 10.Stacy MR, Yu DY, Maxfield MW, Jaba IM, Jozwik BP, Zhuang ZW, et al. Multimodality imaging approach for serial assessment of regional changes in lower extremity arteriogenesis and tissue perfusion in a porcine model of peripheral arterial disease. Circ Cardiovasc Imaging. 2014;7(1):92–9. doi: 10.1161/CIRCIMAGING.113.000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobrucki LW, Dione DP, Kalinowski L, Dione D, Mendizabal M, Yu J, et al. Serial noninvasive targeted imaging of peripheral angiogenesis: validation and application of a semiautomated quantitative approach. J Nucl Med. 2009;50(8):1356–63. doi: 10.2967/jnumed.108.060822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papademetris X, Jackowski AP, Schultz RT, Staib LH, Duncan JS. Computing 3D non-rigid brain registration using extended robust point matching for composite multisubject fMRI analysis. Med Image Comput Comput Assist Interv. 2003;2879:788–95. [Google Scholar]
- 13.Di Francescomarino S, Sciartilli A, Di Valerio V, Di Baldassarre A, Gallina S. The effect of physical exercise on endothelial function. Sport Med. 2009;39(10):797–812. doi: 10.2165/11317750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Moeller S, Yacoub E, Olman CA, Auerbach EJ, Strupp J, Harel N, et al. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med. 2010;63(5):1144–53. doi: 10.1002/mrm.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joshi A, Scheinost D, Okuda H, Belhachemi D, Murphy I, Staib LH, et al. Unified framework for development, deployment and robust testing of neuroimaging algorithms. Neuroinformatics. 2011;9(1):69–84. doi: 10.1007/s12021-010-9092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCallum JC, Lane JS., III Angiosome-directed revascularization for critical limb ischemia. Semin Vasc Surg. 2014;27:32–7. doi: 10.1053/j.semvascsurg.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Bosma J, Dijksman LM, Lam K, Wisselink W, van Swijndregt AD, Vahl A. The costs and effects of contrast-enhanced magnetic resonance angiography and digital subtraction angiography on quality of life in patients with peripheral arterial disease. Acta Radiol. 2014;55(3):279–86. doi: 10.1177/0284185113496560. [DOI] [PubMed] [Google Scholar]
- 18.De Vos MS, Hawkins AT, Hevelone ND, Hamming JF, Nguyen LL. National variation in the utilization of alternative imaging in peripheral arterial disease. J Vasc Surg. 2014;59(5):1315–22. doi: 10.1016/j.jvs.2013.11.059. [DOI] [PubMed] [Google Scholar]