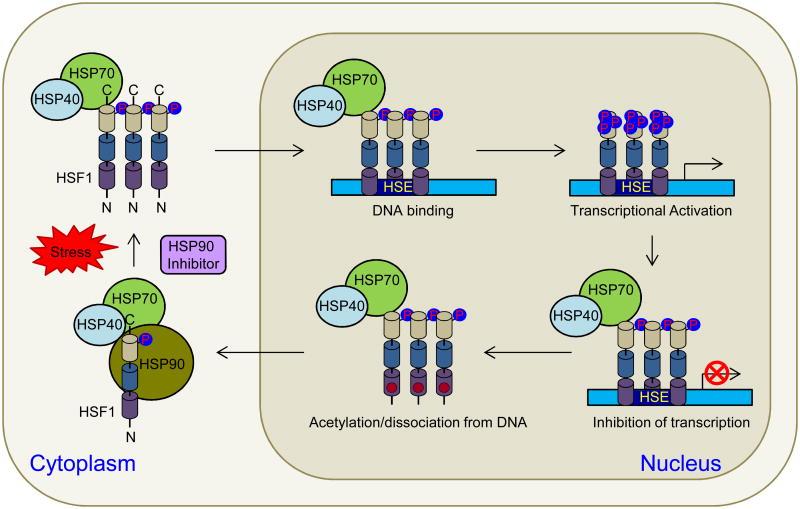
Figure 3.
HSF1 activation and attenuation. HSF1 in unstressed cells exists in the cytoplasm as an inactive monomer whose activity is repressed by interaction with HSP90, HSP70 and HSP40 as well as phosphorylation on S303 and S307 residues. Upon stress or in the presence of HSP90 inhibitors, HSP90 dissociates from HSF1/HSP70/HSP40 complex, allowing HSF1 trimerization and translocation into the nucleus where it binds to heat shock elements (HSE) in the promoters of stress-induced genes. Post-translational modifications, such as phosphorylation and sumoylation, are involved in regulating the transactivation capacity of HSF1. HSF1 attenuation involves negative feedback from HSPs, which represses the transactivation of DNA-bound HSF1 and the acetylation of K80 in the DNA binding domain (DBD), which inhibits HSF1 DNA binding.