Figure 3.
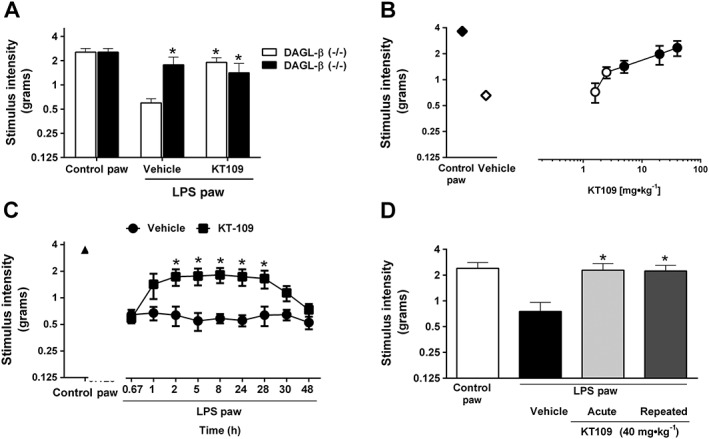
Pharmacological and genetic inhibition of DGLβ blocks LPS‐induced allodynia. (A) LPS‐treated DAGL‐β (−/−) mice do not develop allodynia. KT109 (40 mg·kg−1) reverses LPS‐induced allodynia in wild‐type mice but does not further alter the anti‐allodynic phenotype in DAGL‐β (−/−) mice. n = 13 WT/KO – vehicle groups, n = 14 WT/KO – KT109 groups. (B) KT109 reverses LPS‐induced allodynia in a dose‐dependent manner. For the doses of 1.6, 2.5 and 20 mg·kg−1, n = 5 mice per group, and for the doses of 5, 40 mg·kg−1, n = 6 mice per group. Filled symbols denote P < 0.05 significance from LPS + vehicle. (C) KT109 reversal of LPS‐induced allodynia persists for a long duration of time. n = 6 mice per group. (D) Acute or repeated administration of KT109 (40 mg·kg−1) prevents the expression of LPS‐induced allodynia. For vehicle and acute KT109 groups, n = 5 mice per group, and for the repeated KT109 administration group, n = 6 mice. Data represent mean ± SEM, *P < 0.05 versus WT LPS + vehicle. The differences in experimental numbers within these studies reflect an odd number of animals evenly distributed in the experimental design.
