Figure 7.
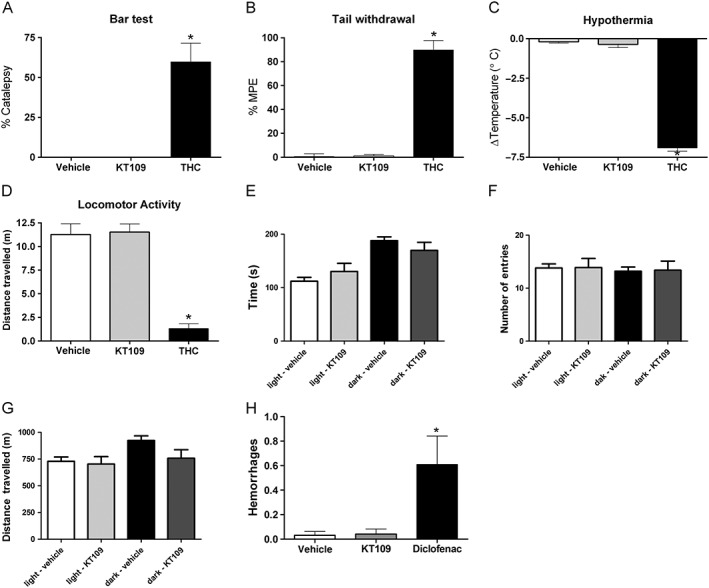
KT109 does not produce appreciable untoward side effects. KT109 (n = 5) does not produce catalepsy (A), antinociception in the tail withdrawal test (B), hypothermia (C) or decreases in locomotion (D). The positive control, THC (n = 5, 30 mg·kg−1) was active in each assay. *P < 0.05 versus vehicle (n = 6). The differences in experimental numbers reflect an odd number of animals evenly distributed in the experimental design. KT109 (40 mg·kg−1, i.p.) does not alter behaviour in the light/dark box assay. (E) KT109 does not alter time spent in either the light or the dark side. (F) KT109 does not reduce the distance travelled. (G) KT109 does not change the number of total entries into the light or dark side. n = 10 mice per group, *P < 0.05 versus vehicle + light side. (H) KT109 (40 mg·kg−1, i.p.) does not produce gastric haemorrhages in food‐restricted mice. Mice treated with the COX‐1/2 inhibitor diclofenac sodium (100 mg·kg−1, i.p.) developed gastric haemorrhages. For the vehicle treated group, n = 9 mice, and for the KT109/diclofenac groups, n = 8. Data represent mean ± SEM, *P < 0.05 versus vehicle. The differences in experimental numbers reflect an odd number of animals evenly distributed in the experimental design.
