Figure 3.
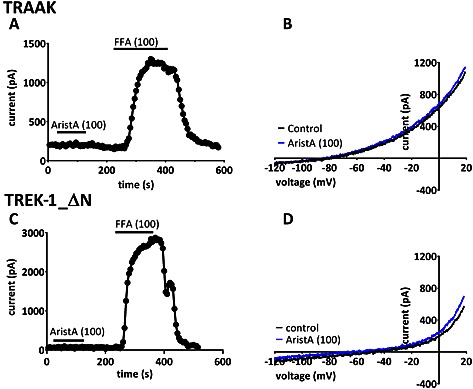
Aristolochic acid (AristA) has no effect on the closely related two‐pore‐domain potassium channel, TRAAK and the N‐terminally truncated TREK‐1 isoform (TREK‐1_ΔN). (A) Time course plot showing lack of effect of AristA (100 μM) on human TRAAK isoform 2 (the sequence of this isoform differs from the canonical sequence by the addition of 26 amino acids, preceding the start codon of isoform 1). (B) TRAAK currents evoked by ramp changes in voltage in control conditions and in the presence of 100 μM AristA. (C) Time course plot showing lack of effect of AristA (100 μM) on the alternative translational initiation isoform of TREK‐1 (TREK‐1_ΔN), where the first 41 amino acids of wild‐type TREK‐1 are missing. (D) TREK‐1_ΔN currents evoked by ramp changes in voltage in control conditions and in the presence of 100 μM AristA.
