Figure 5.
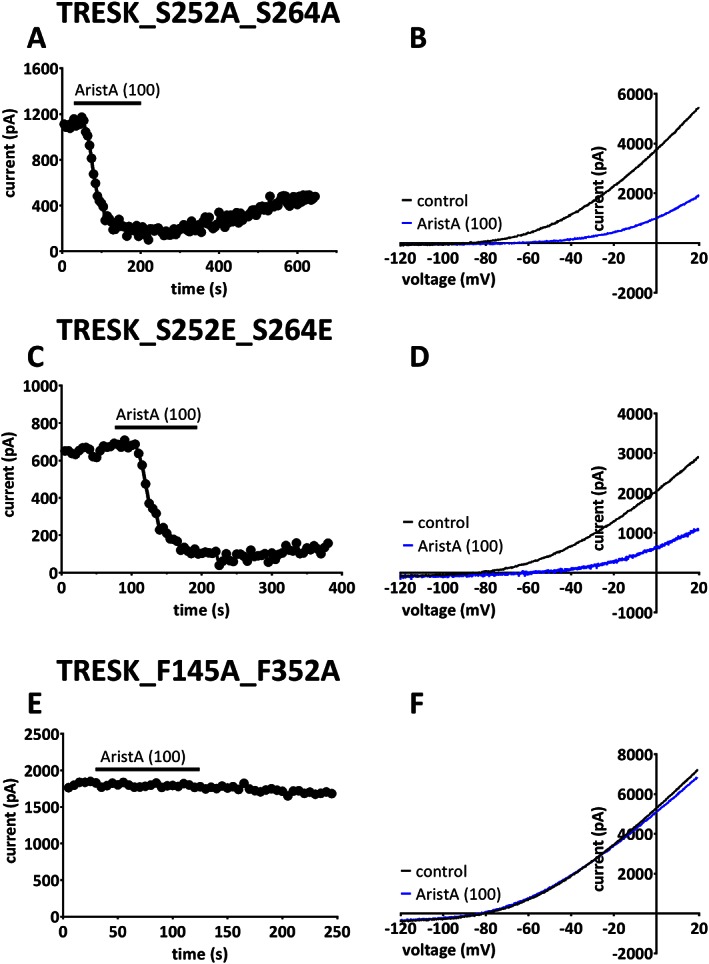
Aristolochic acid (AristA) inhibits serine phosphorylation mutants of TRESK, but its effect is significantly attenuated by the phenylalanine mutants in the inner pore. (A) Time course plot showing an inhibition of the phosphorylation mutant TRESK_S252A_S264A by AristA (100 μM). (B) TRESK_S252A_S264A currents evoked by ramp changes in voltage in control conditions and in the presence of 100 μM AristA. (C) Time course plot showing an inhibition of the phosphorylation mutant TRESK_S252E_S264E by AristA (100 μM). (D) TRESK_S252E_S264E currents evoked by ramp changes in voltage in control conditions and in the presence of 100 μM AristA. (E) Time course plot showing lack of effect on mutant TRESK_F145A_F352A by AristA (100 μM). (F) TRESK_F145A_F352A currents evoked by ramp changes in voltage in control conditions and in the presence of 100 μM AristA.
