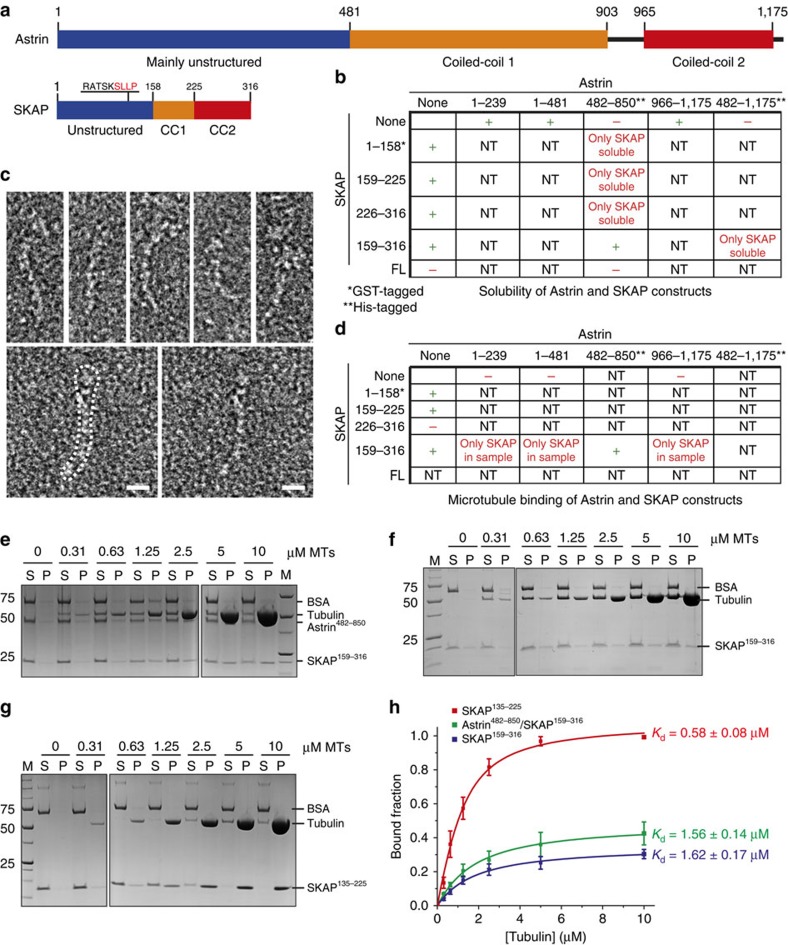
Figure 1. Domain characterization of the SKAP:Astrin complex.
(a) Domain organization of the SKAP:Astrin complex. Both Astrin and SKAP consist of three domains: a mainly unstructured N-terminal domain followed by two coiled-coil domains. (b) Solubility of Astrin and SKAP constructs in isolated and co-expression in bacteria and insect cells. NT, not tested. Samples not marked by asterisks were purified by means of affinity tags that were later removed proteolytically, as discussed in Methods section. (c) Representative electron micrographs of negative-stained SKAP159–316:Astrin482–850 complex. Scale bar, 10 nm. (d) Summary of microtubule-binding properties of Astrin and SKAP constructs in co-sedimentation assays with purified isolated or combined constructs. NT, not tested. (e) Representative SDS–PAGE of microtubule co-sedimentation assay with 0-10 μM taxol-stabilized microtubules (MTs) and 1 μM SKAP159–316:Astrin482–850 complex. M, molecular weight marker; P, pellet fraction; S, soluble fraction. Images originate from two different gels as indicated by dividing lines. (f) Representative SDS–PAGE of microtubule co-sedimentation assay with 0-10 μM taxol-stabilized MTs and 1 μM SKAP159–316. M, molecular weight marker; P, pellet fraction; S, soluble fraction. Images originate from two different gels as indicated by dividing lines. (g) Representative SDS–PAGE of microtubule co-sedimentation assay with 0-10 μM taxol-stabilized MTs and 1 μM SKAP135–225. M, molecular weight marker; P, pellet fraction; S, soluble fraction. Images originate from two different gels as indicated by dividing lines. (h) Quantification and fitting analysis of microtubule co-sedimentation assays shown in (e–g; mean±s.e.m., n≥3).