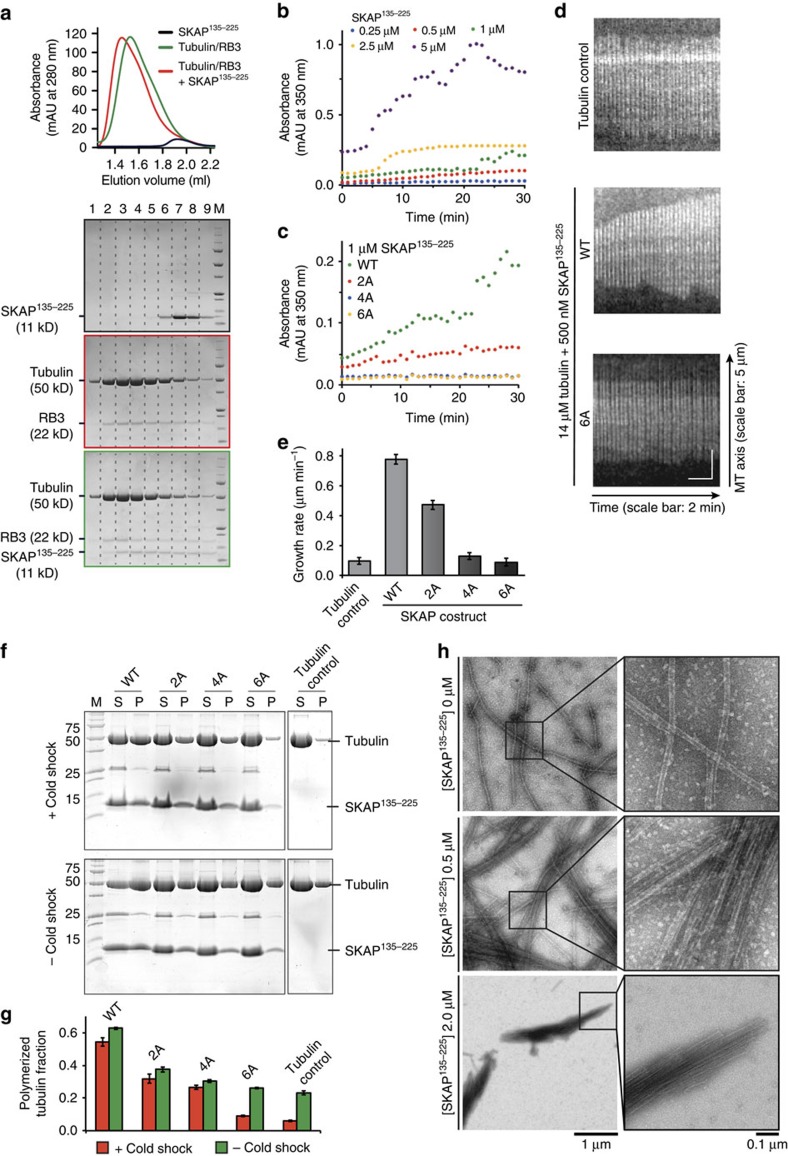
Figure 4. SKAP135–225 binds to tubulin in solution and promotes its polymerization and bundling in vitro.
(a) Representative chromatogram and SDS–PAGE of analytical size-exclusion migration shift assays with SKAP135–225 and tubulin/RB3 complex on a Superdex 200 5/150. (b) In vitro Tubulin polymerization assays with 5 μM tubulin and 0.25-5 μM SKAP135–225 (n=4). Tubulin polymerization was followed via absorbance at 350 nm. (c) In vitro tubulin polymerization assays with 5 μM tubulin and 1 μM SKAP135–225 wild type (WT) or indicated mutants (n≥3). Tubulin polymerization was followed via absorbance at 350 nm. (d) Kymographs of microtubules observed in TIRF experiments in absence or presence of 500 nM SKAP135–225 WT or 6A mutant. Additional kymographs for tubulin control, WT and mutants are shown in Supplementary Fig. 4A. (e) Microtubule growth rates observed in TIRF experiments in absence or presence of 500 nM SKAP135–225 WT or indicated mutants (mean±s.e.m., n=3, for each condition ≥15 microtubules were analysed). (f) Representative SDS–PAGE of microtubule cold shock assay (top) and control without cold treatment (bottom) in absence or presence of SKAP135–225 WT or indicated mutants. M, molecular weight marker; P, pellet fraction; S, soluble fraction. Images originate from two different gels each as indicated by dividing lines. (g) Quantification of microtubule cold shock assay shown in f (mean±s.e.m., n=3). (h) Representative electron micrographs of negative-stained taxol-stabilized microtubules in the presence of 0-2 μM SKAP135–225.