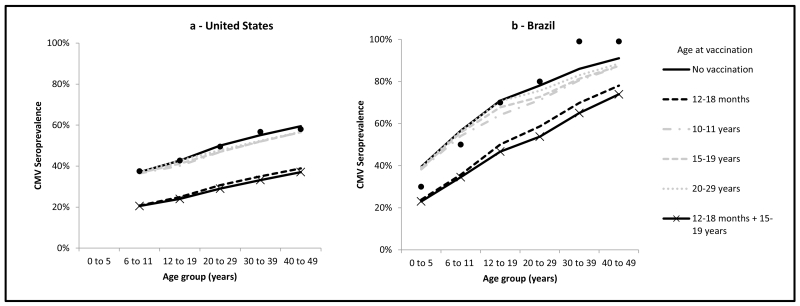
Figure 2.
Impact of vaccination on CMV seroprevalence from natural infection by age group at equilibrium, assuming different ages at vaccination, age-specific duration of infectiousness, 20 year duration of latency, and a vaccine with 70% efficacy, 90% coverage and 5-year duration of protection, United States and Brazil.
Black dots indicate available CMV serological data; U.S. data are from the 1999-2004 National Health and Nutrition Examination Survey [15], data for the age group 0-5 years were not available; Brazilian data are from CMV serological data from Caieiras, Sao Paulo, 1990-1991 [16].