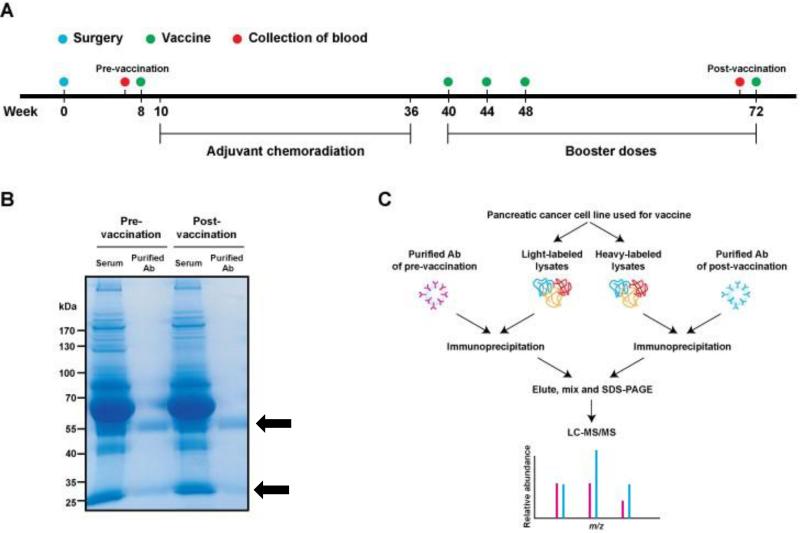
Figure 1. Overview of the study design and SASI screen.
(A) Vaccination scheme based on Lutz et.al (6). The date of surgery was set as a start date for vaccination schedule (i.e. week 0). First vaccine was administered at week 8 prior to the adjuvant chemoradiation period (week 10 to week 36). Three consecutive booster injections of the vaccine were carried out at 40th, 44th and 48th weeks and then, last vaccine was administered at 72nd week. Blood samples collected before the first injection and before the last injection were used for the SASI approach. (B) Purification of serum antibodies. Serum antibodies from pre- and post-GVAX of patients with favorable DFS > 3 years were isolated with a protein G affinity column. Coomassie blue staining of the SDS-PAGE gel shows high purity of antibody fragments (black arrows) from serum samples and no significant difference was observed in the antibody amounts between pre- and post-vaccination serum samples. (C) Experimental scheme. One of the GVAX pancreatic cancer cell line was labeled by the SILAC method to produce light-labeled cell lysates and heavy-labeled cell lysates, each of which was then incubated with purified pre- and post-vaccination antibodies, respectively, for separate immunoprecipitations. Immunoprecipitated light- and heavy-labeled proteins were combined and separated on a SDS-PAGE gel. Peptides extracted from the gel were analyzed by LC-MS/MS.