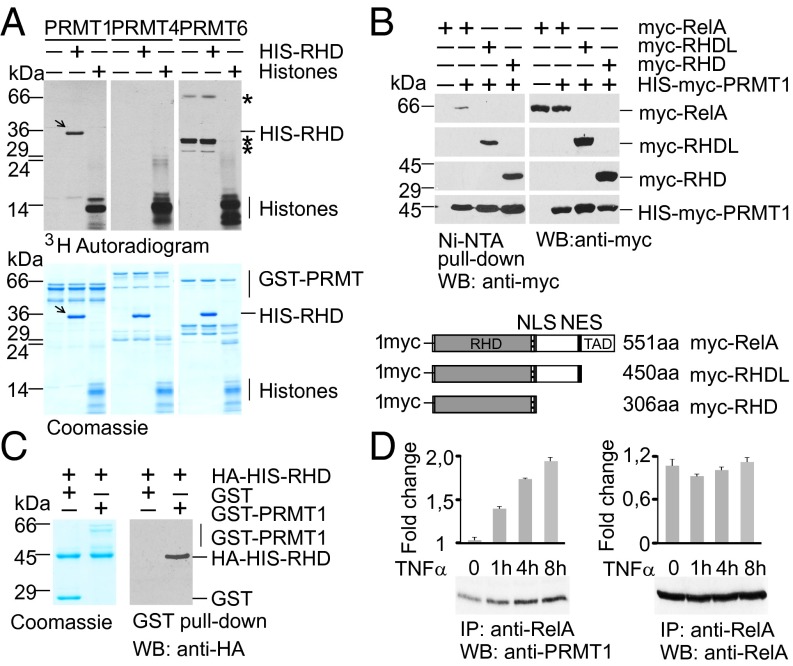
Fig. 1.
PRMT1 interacts with the RelA subunit of NF-κB and methylates the RHD of RelA. (A) Recombinant PRMTs were incubated with histones or His-RelA (amino acids 1–306) in the presence of 3H-SAM, resolved by SDS/PAGE, and analyzed by autoradiography. Arrows indicate methylated RelA; asterisks represent automethylated PRMT6 peptides. (B) PRMT1 forms a complex with RelA in vivo. (Upper) Full-length and deletion variants of RelA were expressed in HEK 293T cells and copurified with ectopic His-PRMT1. (Lower) The variants of RelA are shown schematically. The RHD, TAD, and nuclear import (NLS) and export (NES) signals are indicated. (C) PRMT1 physically binds to the RHD of RelA. In vitro GST pull-down assays were performed using the HA-His-RHD with GST or GST-PRMT1 purified from bacteria. The precipitated proteins were analyzed by immunoblotting with anti-HA antibody. (D) HEK 293T cells were stimulated with 20 ng/mL of TNFα for the indicated times. Endogenous RelA complexes were immunoprecipitated from the cell lysates and analyzed for the presence of PRMT1. Intensities of the immunoblot signals were quantified using ImageJ 1.49.