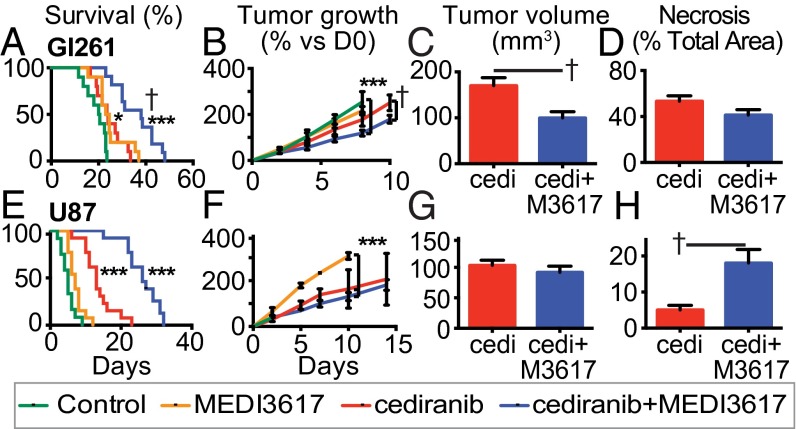
Fig. 1.
Dual cediranib+MEDI3617 therapy enhances survival and reduces tumor burden in Gl261 and U87 tumors compared with cediranib therapy alone. Mice bearing Gl261 (A–D) or U87 (E–H) tumors were treated with control (green traces), MEDI3617 (orange traces), cediranib (red traces/bars), or dual therapy (blue traces/bars). (A) In Gl261 tumors, both cediranib and MEDI3617 monotherapies led to significantly higher overall median survival (24 d) than control treatment (20 d) (cediranib *P = 0.017; MEDI3617 *P = 0.011; n = 10). Dual therapy (n = 11) led to a significantly higher median survival (38 d) than control (***P < 0.0001) or cediranib treatment (†P = 0.002). (B) There was a significant difference in the growth rate of tumors treated with dual therapy compared with both control-treated (***P < 0.0001) and cediranib-treated (†P = 0.0076) tumors as measured by OFDI. (C) Dual therapy-treated tumors were significantly smaller than cediranib-treated tumors at day 20 as measured by MRI (†P = 0.0089). (D) There was no change in the extent of necrosis at day 20 (P = 0.11). (E) In the U87 model, both cediranib and dual therapy-treated mice had a significantly higher overall median survival (26 d and 13 d, respectively; n = 13) than control-treated mice (5 d; n = 12; ***P < 0.0001). (F and G) There was no difference in tumor growth (F) or volume (G) between dual therapy-treated tumors and cediranib-treated tumors in the U87 model. (H) There was a significant increase in ischemic hypoxic changes (early necrosis) in dual therapy-treated tumors compared with cediranib-treated tumors at day 6 (†P = 0.030). cedi, cediranib; cedi+M3671, cediranib+MEDI3617. Error bars represent the SEM. *P < 0.05, **P < 0.01, ***P < 0.001 compared with control unless otherwise indicated.