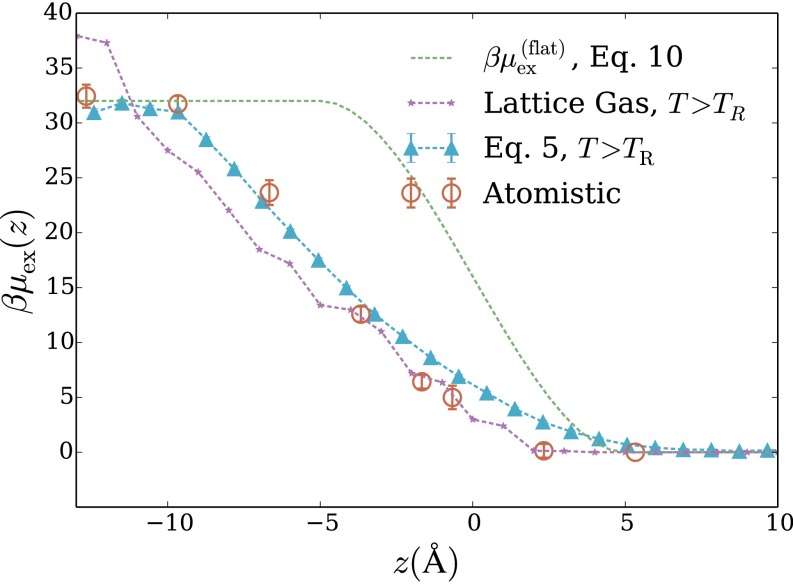
Fig. S2.
Excess chemical potential of a spherical hydrophobic solute as a function of its perpendicular displacement z from the air–water interface. corresponds to bulk vapor, to bulk liquid, and to the Gibbs dividing surface between the two coexisting phases. Data are shown for atomistic simulations, for the LCW-inspired coarse-grained model of Eq. 5 (with parameters Å), and for the estimate in Eq. 10 based on a completely quiescent interface. This solute excludes the center of each water molecule from a sphere of radius Å (as shown in the inset schematic). In addition, we show data for an Ising lattice gas with Because it is not possible to implement truly spherical solute in the simple lattice gas system, we used a cubic solute with side length 7.24 Å, so that the surface area is equal to that of the Å sphere.