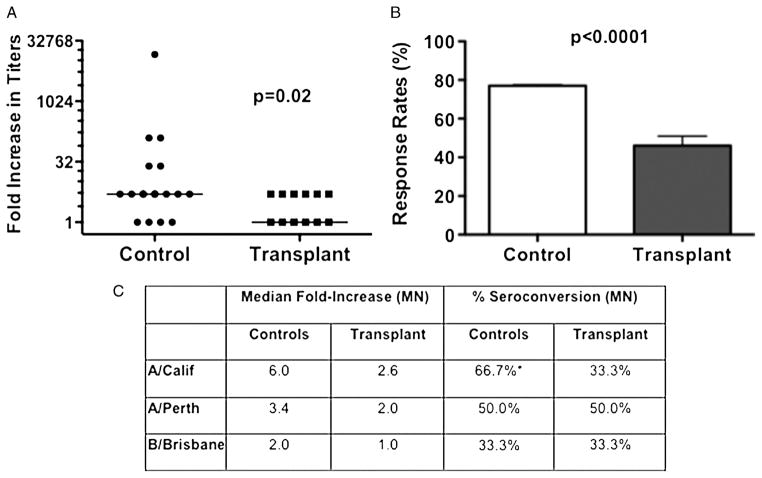
FIGURE 3.
Anti-influenza IgG seroresponse and seroconversion following influenza vaccination. A, quantification of the anti-influenza IgG seroresponse by ELISA. Data represent the increase in the geometric mean titers (GMT; bar indicates median increase) from day 0 to day 14/28, with transplant patients (N=13) exhibiting significantly reduced responses compared to healthy controls (N=18). B, the response rate (%), which refers to the percentage of subjects with a 4-fold increase in titers on day 14/28 post-vaccination, was also significantly different between the two groups. C, anti-influenza titers were quantified by microneutralization (MN) assays to A/California/7/09-like (H1N1) (A/Calif), A/Perth/16/2009-like (H3N2) (A/Perth), and B/Brisbane/60/2008-like (B/Brisb) influenza virus. Data are presented as the median fold increase and percent seroconversion, which is defined as the percentage with a 4-fold increase in titers at day 28 post-vaccination. Data are from transplant patients (N=6) or controls (N=12) vaccinated in 2011. Statistical significance was determined by Mann-Whitney test (A) or unpaired t test (B) or two-way ANOVA with a Bonferroni post hoc test (C).