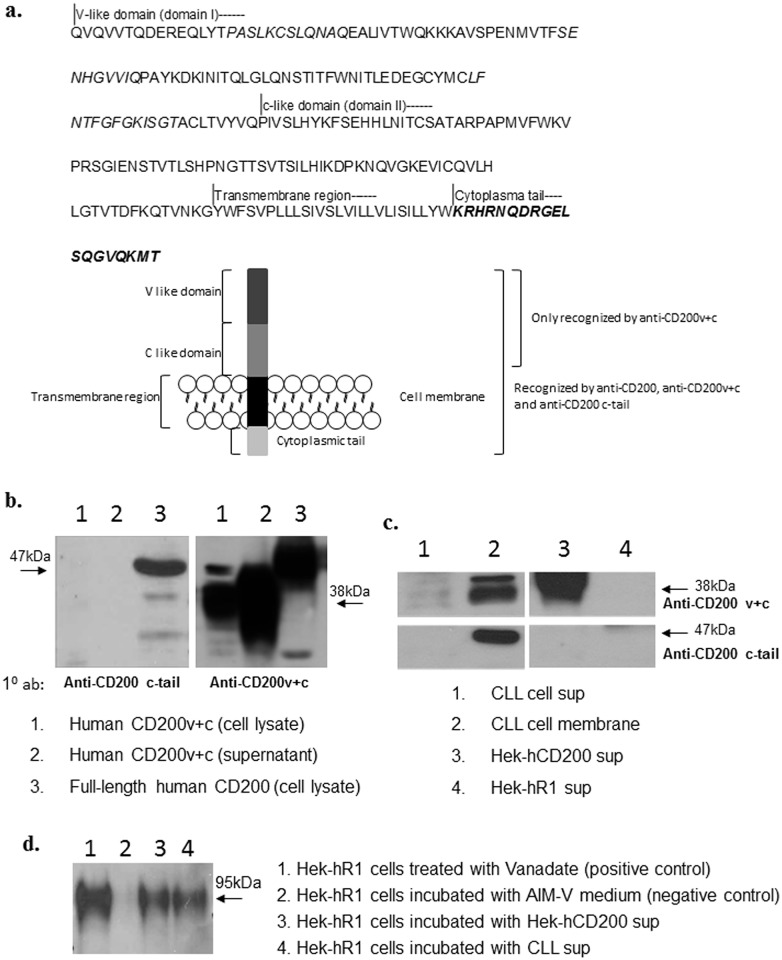
Fig 6. Absence of epitopes associated with the cytoplasmic domain of CD200 in sCD200 and functional properties of sCD200.
a) Amino acid sequence of full-length human CD200. The peptide sequence used for generation of a rabbit anti-CD200 cytoplasmic-tail antibody is highlighted in bold. The diagram showed proteins detected in Fig 6b-d, and the antibodies used to detect them. b) Characterization of rabbit anti-CD200 c-tail serum; the antiserum recognized lysates from Hek-hCD200 cells (lane 3), which expressed full-length CD200, but not lysates or supernatant from Hek293 cells transfected with hCD200v+c (lane 1 and lane 2), indicating specificity for the cytoplasmic domain of CD200. However, they were all recognized by anti-CD200v+c antibodies. c) Membrane extracts from CLL cells were recognized by both rabbit anti-hCD200v+c serum and rabbit anti-CD200 c-tail serum (lanes 2). sCD200 immunoprecipitated from CLL (lanes 1) and Hek-hCD200 (lane 3) supernatants was recognized only by rabbit anti-hCD200v+c serum, but not by rabbit anti-CD200 c-tail serum, indicating that sCD200 released from both cell types lacked the cytoplasmic domain of CD200. Immunoprecipitates from Hek-CD200R1 transfected cells were, as expected, not recognized by either anti-CD200v+c or anti-CD200 c-tail serum (lane 4). d) Hek-CD200R1 cells were immunoprecipitated with anti-phosphotyrosine antibody specific for the phosphorylated tyrosine in the CD200R1 tail [23] following incubation with sCD200-containing CLL and Hek-hCD200 supernatants. Products were subsequently run on 10% SDS-PAGE gel and probed with the rabbit anti-hCD200R1 serum. CD200R1 was phosphorylated by both CLL (lane 4) and Hek-hCD200 (lane 3) supernatants, but not AIMV medium (lane 2), consistent with the hypothesis that sCD200 was capable of binding to, and causing phosphorylation of CD200R1.