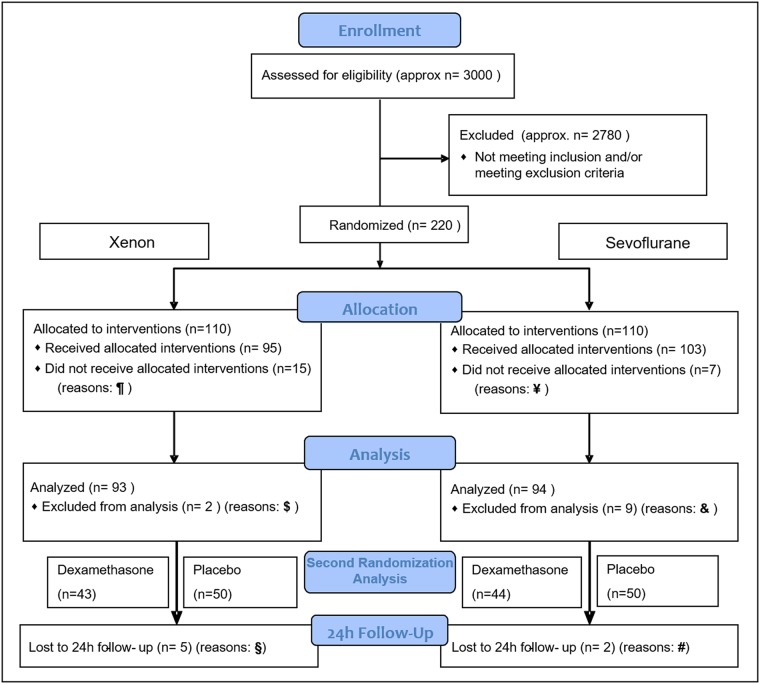
Fig 2. Consort flow chart.
A detailed flow chart depicts all patients enrolled in the trial including the reasons for study drop-out. 220 patients in total were randomized after written informed consent into one of the same-sized study groups. Pre-interventional drop out in the xenon group occurred in fifteen cases (¶): Five patients withdrew their consent; five were excluded after randomization for safety reasons. Two patients did not receive the scheduled surgery; three did not receive the allocated intervention for administrative reasons. Post-interventional drop-out in the xenon group occurred in two cases ($) by exclusion from data analysis due to study protocol violation. 93 patients in the xenon group received the allocated intervention and data analysis. Of these patients, 43 received dexamethasone as randomized prophylactic treatment, whereas 50 received placebo. Five patients (§) withdrew preterm because of severe nausea. Their data until the time of dropout was included into the final analysis. Pre-interventional drop-out in the sevoflurane group occurred in seven cases (Ұ): One patient withdrew his consent; two were excluded after randomization for safety reasons. Two patients did not receive the scheduled surgery; two did not receive the allocated intervention for administrative reasons. Post interventional drop-out in the sevoflurane group occurred in nine cases (&): One patient dropped out due to a serious adverse event not associated with the study intervention. Eight patients of the sevoflurane group were excluded from data analysis because of violation of the study protocol. 94 patients in the sevoflurane group received the allocated intervention and data analysis, with 44 cases of dexamethasone as randomized prophylactic treatment and 50 placebo. Two patients (#) did not complete the 24h-follow-up because of withdrawal due to severe nausea. Their data until the time of dropout was included into the final analysis.