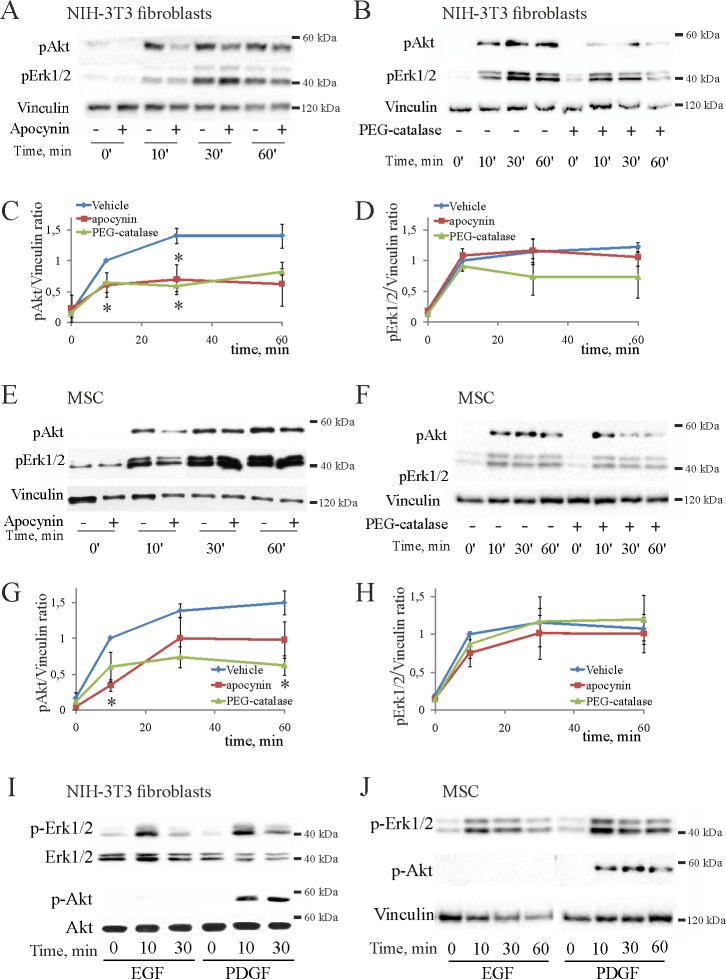
Fig 4. PDGF stimulates redox-sensitive phosphorylation of PKB/Akt in mesenchymal cells, whereas EGF has no effect.
(A)–(B), representative western blots showing the effects of apocynin (A) or PEG-catalase (B) on phosphorylation kinetics of PKB/Akt and Erk1/2 in PDGF-stimulated 3T3 fibroblasts, and vinculin staining in the same lysates used for the loading control. (C)–(D), the corresponding changes in phosphorylation of PKB/Akt or Erk1/2 in fibroblasts analyzed in 4 independent experiments by normalization of phosphorylation signals exemplified above to the vinculin content. Additionally, each data set was normalized to the value of 10 min stimulation in uninhibited control, which therefore has no error bar; (*) p < 0.05 as compared to uninhibited controls. (E)–(F), representative western blots showing the effects of apocynin (E) or PEG-catalase (F) on phosphorylation kinetics of PKB/Akt and Erk1/2 in PDGF-stimulated MSC, and vinculin staining in the same lysates used for the loading control. (G)–(H), the corresponding changes in phosphorylation of PKB/Akt or Erk1/2 in MSC analyzed in 4 independent experiments by normalization of phosphorylation signals exemplified above to the vinculin content. As above, each data set was normalized to the value of 10 min stimulation in uninhibited control; (*) p < 0.05 as compared to uninhibited controls. (I)–(J), PDGF, but not EGF stimulates phosphorylation of PKB/Akt in 3T3 fibroblasts (I) and MSC (J), but both PDGF and EGF similarly stimulate phosphorylation of Erk1/2. Shown are representative membranes from 2 independent experiments.