Figure 2.
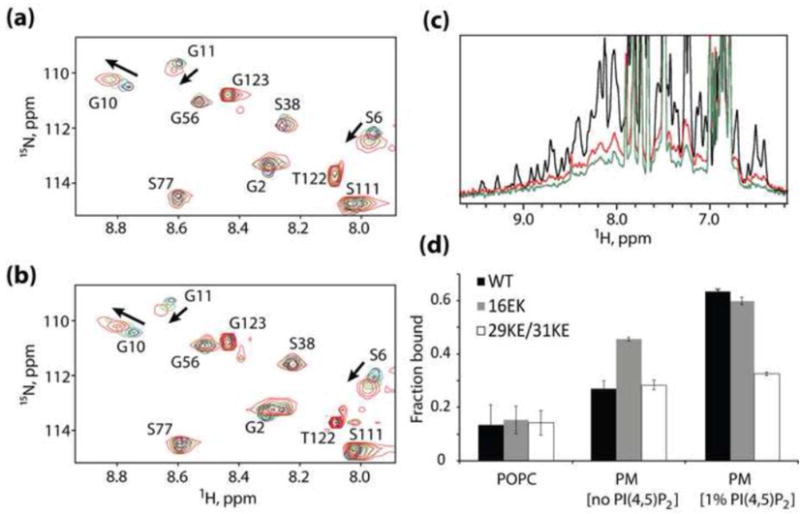
Mechanism of enhanced membrane binding by 16EK. (a,b) Concentration-dependent NMR chemical shift changes observed for residues S6, G10 and G11 in 1H-15N HSQC spectra of the wild-type (a) and 16EK (b) myrMA proteins. Spectral changes reflect a myristyl switch equilibrium switch from myristate-sequestered (low concentration) to myristate-exposed (high concentration) species [13], and indicate that the 16EK mutation does not perturb the myristyl switch. (c) One-dimensional 1H NMR spectra obtained for the myr-16EK mutant under various conditions: in the absence of liposomes (black) and in the presence of membrane mimetic liposomes that lack (red) or contain (green) PI(4,5)P2 (1%). Signal losses correlate with increasing fractions of liposome-bound protein. (d) Fractions of protein bound to POPC liposomes (left) and to membrane mimetic liposomes in the absence (center) and presence (right) of PI(4,5)P2 (1%), as determined by 1D 1H NMR. The wild type, 16EK, and 29KE/31KE proteins bind poorly to POPC liposomes but exhibit differentially enhanced binding to liposomes with PM-like compositions.
