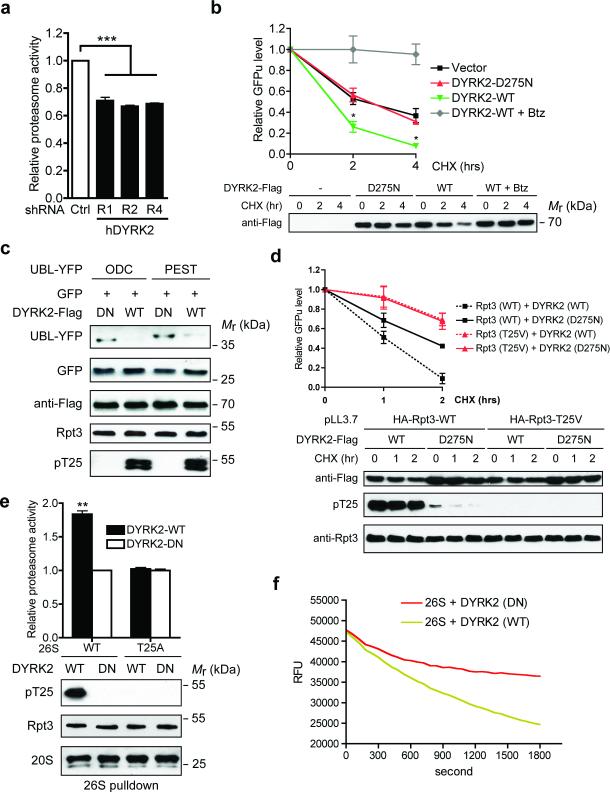
Figure 5. DYRK2 is a positive regulator of proteasome activity.
(a) Proteasome activity in total cell lysates from 293T cells stably expressing control or DYRK2 shRNAs was measured using Suc-LLVY-AMC as substrate. ***p<0.001 (One-way ANOVA, mean ± s.e.m from n=3 independent experiments)..
(b) DYRK2 promotes GFPu degradation. 293T cells were co-transfected with vector control, DYRK2-WT or DYRK2-D275N and GFPu. After 1-hour pre-treatment of DMSO or 1 μM Bortezomib (Btz), cycloheximide (CHX, 50 μg/ml) was added for 0, 2 or 4 hours. GFP fluorescence in cell lysates was determined at each time point, background-subtracted and normalized to the starting level at time 0. *p<0.05 (DYRK2-WT vs. Vector or DYRK2-DN, One-way ANOVA, mean ± s.e.m from n=3 independent experiments). Expression of the DYRK2-3xFlag-V5 constructs was determined by anti-Flag western blot.
(c) DYRK2 promotes the degradation of additional proteasome reporters. 293T cells were co-transfected with the indicated constructs as in (b) and cell lysates were analyzed by western blot.
(d) DYRK2 promotes GFPu degradation in a T25-dependent manner. 293T cells stably expressing HA-Rpt3-WT or T25V was transfected and treated with CHX as in (b). GFPu levels are shown as mean ± s.e.m from n=3 independent experiments (top). DYKR2 expression and T25 phosphorylation were confirmed by western blot (bottom). Note that no T25 phosphorylation was detected in 293T pLL3.7-HA-Rpt3-T25V cells even in the presence of overexpressed DYRK2-WT, indicating a complete switch from endogenous WT Rpt3 to HA-Rpt3-T25V.
(e) DYRK2 activates wild-type proteasome in vitro via T25 phosphorylation. 26S proteasomes were purified from parental and T25A MDA-MB-468 cells in the absence of phosphatase inhibitors, followed by in vitro phosphorylation with DYRK2. After removal of DYRK2, proteasome activity was measured with Suc-LLVY-AMC (top, **p<0.01, two-tailed paired Student's T-test, mean ± s.e.m from n=3 independent experiments). Total Rpt3, T25 phosphorylation and 20S subunits from the pulldown are shown by western blot (bottom).
(f) In vitro degradation of polyubiquitinated GFP-titinV15P-cyclin-PY fusion protein by proteasomes treated with DYRK2-WT or D275N (in triplicates). RFU, relative fluorescence units.
Source data for a, b, d and e can be found in Supplementary Table 3.