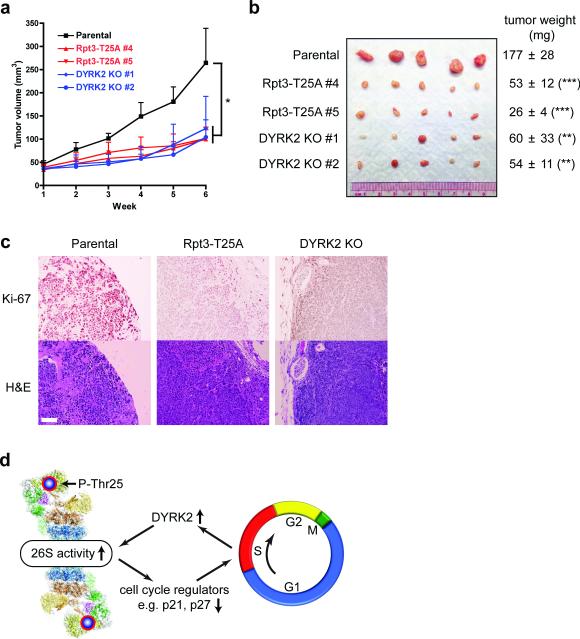
Figure 8. Rpt3-T25 phosphorylation is required for tumor growth in vivo.
(a) Tumor xenograft studies with parental and genome-edited MDA-MB-468 cells injected subcutaneously into nude mice. Tumor volumes at each time point after injection are shown (mean ± s.e.m). *p<0.05 (n = 5 mice, One-way ANOVA).
(b) Xenograft tumors from (a) were resected at 6 weeks post-injection and imaged. Tumor weights are shown on the right as mean ± SEM. **p<0.01, ***p<0.001 (n =5 mice, compared to parental line, two-tailed non-paired Student's T-test).
(c) Histological examination of consecutive sections of the tumors with Ki-67 and hematoxylin/eosin staining. Scale bar = 100 μm.
(d) A model of reversible phospho-regulation of the 26S proteasome (adapted from ref.53). The approximate position of Rpt3-T25 in the 26S proteasome complex is highlighted. Cell cycle-dependent Rpt3-T25 phosphorylation regulated by DYRK2 facilitates the degradation of key proteins such as p21 and p27, which in turn promotes cell cycle transition. Pharmacological intervention of this process by targeting proteasome kinases can have therapeutic potentials.