STRUCTURED ABSTRACT
Objective
Define gut associated lymphocyte phenotype (GALT) changes with parenteral nutrition (PN) and PN with bombesin (BBS).
Summary Background Data
PN reduces respiratory tract (RT) & GALT Peyer’s patch and lamina propria (LP) lymphocytes, lowers gut and RT IgA levels and destroys established RT antiviral & antibacterial immunity. BBS, an enteric nervous system (ENS) neuropeptide, reverses PN-induced IgA and RT immune defects.
Methods
Exp 1: IV-cannulated ICR mice received Chow, PN or PN + BBS injections for 5 days. LSR-II flow cytometer analyzed PP and LP isolated lymphocytes for homing phenotypes (L-selectin+ & LPAM-1+) and state of activation (CD25+, CD44+) in T (CD3+) cell subsets (CD4+ & CD8+) along with homing phenotype (L-selectin+ & LPAM-1+) in naive B (IgD+) and antigen-activated (IgD− or IgM+) B (CD45R/B220+) cells. Exp 2: Following initial experiment 1 protocol, LP T regulatory (Treg) cell phenotype was evaluated by Foxp3 expression.
Results
Exp 1: PN significantly reduced LP 1) CD4+CD25+ (activated) and 2) CD4+CD25+LPAM-1+ (activated cells homed to LP) T cells while PN-BBS assimilated Chow levels. PN significantly reduced LP 1) IgD+ (naïve), 2) IgD-LPAM+ (antigen-activated homed to LP) and CD44+ memory B cells while PN-BBS assimilated Chow levels. Exp 2: PN significantly reduced LP CD4+CD25+Foxp3+ Treg cells compared to Chow mice while PN+BBS assimilated Chow levels.
Conclusions
PN reduces LP activated and regulatory T cells as well as naïve and memory B cells. BBS addition to PN maintains these cell phenotypes, demonstrating the intimate involvement of the ENS in mucosal immunity.
INTRODUCTION
Parenteral Nutrition (PN) prevents progressive malnutrition in patients unable to take adequate nutrition via the GI tract. However, compared to enteral nutrition (EN), PN increases the risk of pneumonia and intra-abdominal abscesses, especially pneumonia, in severely injured trauma patients.1–5 The reason for this impairment with PN is multi-factorial but experimental evidence implicates an impairment of mucosal immunity.
The main strategic adaptive component of the mucosal immune system is immunoglobulin A (IgA), a molecule which functions to prevent attachment of bacteria to mucosal surfaces and as well as control intra-luminal bacterial populations. The integrity of mucosal immunity and levels of IgA in both intestinal fluid and the lung depends upon enteral stimulation and feeding. 6–8 When compared to EN, PN with decreased enteral stimulation (PN/DES) in mice decreases both the absolute number of mucosal immune T & B cells and levels of IgA by 50–60% in both the lung and the gut. 6 These changes occur due to effects on T & B cell distribution, T & B cell phenotypes, chemokines, Th2-type cytokines, IgA production and IgA transport as listed in Table 1. 6–12 These effects with PN destroy established antibacterial and antiviral respiratory immunity in the mouse. 11, 13, 14 The relevance of the murine finding to the human condition have been strengthened through comparisons of intestinal immunity changes after PN 15 and in airway responses of the of mice and humans after injury. These experimental observations provide a cogent explanation for the increased risk of infectious in lungs of PN-fed patients.
Table 1.
Alterations in mucosal immunity associated with parenteral nutrition
| Distribution of T&B cells |
| Lower mucosal addressin cellular adhesion molecule-1 expression on Peyer’s patch venules |
| Reduces entry of T&B cells into mucosal immune system for priming |
| Lower chemokines levels in Peyer’s patches and lamina propria and lung |
| Lesser stimulus for cell migration our of vascular tree and into tissue |
| T&B cell phenotypes in Peyer’s patches and lamina propria LUNG |
| CD4+ cells reduced by 50–60% |
| CD8+ cells unchanged |
| Lower levels of Th-2 type cytokines: IL-4 and IL-10 |
| Peyer’s patches: IL-4 stimulates MadCAM-1 expression via NFkB |
| Lamina propria: IL-4 and IL-10 stimulate IgA production by B cells |
| Reduced levels of polymeric immunoglobulin receptor (pIgR) on the mucosal cells |
| pIgR transports IgA from lamina propria to lumen converting it to secretory IgA |
The enteric nervous system (ENS) appears to be intimately associated with the mucosal immune system. The ENS forms a vast network of neurons throughout the gastrointestinal tract with an estimated 3 m of nerve per cm3 of gastrointestinal tissue with most fibers located within 13 micrometers of the mucosa. Neuropeptides synthesized by the ENS regulate gut motility and secretion, mucosal growth and immune function defenses. 16–20 One neuropeptide released in humans soon after ingestion of food is gastrin-releasing peptide (GRP). GRP shares an identical 7-amino acid carboxyl terminus to bombesin (BBS), a neuropeptide originally isolated from the skin of the frog Bombina bombina. BBS is frequently used to study GRP function due to their similar receptor interactions. 16 BBS (and GRP) stimulates the release of many gastrointestinal hormones including gastrin, CCK, and neurotensin. 16, 18 Our lab previously characterized the effects of BBS supplementation with PN (PN+BBS) on mucosal immune defenses. In our model, BBS preserves lymphocyte cell mass in the gut associated lymphoid tissue (GALT), including T & B lymphocytes in Peyer’s patches and the lamina propria and restores mucosal IgA levels in the lungs and the gut. 13, 21–23 BBS also restores established antibacterial and antiviral activity lost during PN. 11, 21
PN/DES reduces the absolute number of T & B cells in the lamina propria, lung and Peyer’s patches with a reduction of CD4+ cells and a reduced CD4/CD8 ration in the lamina propria. Detailed changes of specific lymphocyte phenotypes in mucosal immune sites such as those listed in Table 2 are lacking. In this work we hypothesized that PN/DES decreases lymphocyte phenotypes important in homing, activation, immunologic memory and IgA production. In addition, we hypothesized that the administration of BBS during PN treatment reverses these phenotype changes. We believe that by studying the effect of PN/DES on mucosal immunity and the role of the enteric nervous system in regulating this process allows better understanding mucosal immunity changes associated with injury and critical care management. Since some patients inevitably have prolonged contraindications to EN and require PN to avoid progressive protein-calorie malnutrition, this work may allow the development of therapies which correct these deficits and alleviate some infectious complications associated with PN/DES.
Table 2.
Lymphocyte Surface Markers with Corresponding Phenotypes and Descriptions
| Surface markers | Phenotype | Description |
|---|---|---|
| CD3 | T cells | pan T cell marker |
| CD4 | TH cells | helper T cells – humoral immunity |
| CD8 | TC cells | cytotoxic T cells – cell-mediated immunity |
| foxp3 | T regulatory cell | T cell regulating immune responses |
| CD45/B220 | B cells | Pan B cell marker |
| IgD | Naïve B cell | B cell unexposed to antigen |
| CD62L | L-selectin | naïve lymphocyte homed to inductor site |
| LPAM-1 | α4β7 integrin | activated lymphocyte homed to effector site |
| CD25 | activation | regulatory/suppressor cells |
| CD44 | memory | effector/memory cells |
MATERIALS AND METHODS
Animals
Male six-to-eight-week-old Institute of Cancer Research mice were purchased from Harlan (Indianapolis, IN) and housed in the Animal Research Facility of the William S. Middleton Memorial Veterans Hospital, an American Association for Accreditation of Laboratory Animal Care accredited conventional facility. Mice were allowed to acclimatize for 1 week with free access to standard chow diet (PMI Nutritional International, St. Louis, MO) and water, under controlled conditions of temperature and humidity with a 12:12 hour light:dark cycle.
Experiment 1
Intravenous cannulation and nutrition
Thirty four mice were randomized to three diet groups (chow, n = 10; PN, n = 12, PN+BBS, n = 12), anesthetized with an intraperitoneal ketamine (100 mg/kg) and acepromazine (5 mg/kg) mixture and cannulated via the right external jugular vein (0.012-in ID/0.25-in OD; Helix Medical, Inc, Carpinteria, CA). Catheters were tunneled subcutaneously over the back and exited midtail. Mice were immobilized by the tail, which has been shown not to induce significant physical or biochemical stress 24, 25.
After catheterization, mice were connected to infusion pumps and recovered for 48 hours while receiving 4 mL of 0.9% saline/day, as well as chow and water ad libitum. After the recovery period, the two different diets were initiated. Chow animals received 0.9% saline at 4 mL/d, as well as chow and water ad libitum throughout the study. Parenterally fed mice received solution at 4 mL/d (day 1), 7mL/d (day 2) and 10 mL/d (days 3–5). The PN solution contained 6.0% amino acids, 35.6% dextrose, electrolytes, and multivitamins, with a non-protein calorie/nitrogen ratio 127.68 Kcal/g nitrogen. In addition, each group received IV injections three times a day (7:00 am, 3:00 pm, and 11:00 pm). Chow and PN fed mice received 100 uL of vehicle saline while the PN+BBS (Sigma, St. Louis, MO) group received 15 ug/kg TID in 100 uL. The feedings met the calculated nutrient requirements of mice weighing 25–30 g 26.
Tissue Harvest and Cell Isolation
After receiving the respective diets for five days mice were disconnected from their infusion pumps and anesthetized with an intraperitoneal ketamine (100 mg/kg) and acepromazine (5 mg/kg) mixture and sacrificed via transection of the left axillary artery.
Peyer’s patch analysis
The peritoneal cavity was opened via a midline incision and the small intestine (SI) was removed from the pylorus to the terminal ileum by dissecting off the mesentery. The SI was flushed with 20 mL of cold CMF-HBSS (500 mL calcium-magnesium-free-hank’s balanced saline solution + 50000 U penicillin/50 mg streptomycin (Sigma, St. Louis, MO)). The peyer’s patches (PP) from the entire length of the SI were removed into 1.5 mL tubes of CMF-HBSS. PP were strained through 100-μm mesh with a total volume of 15 mL CMF-HBSS. The effluent was collected and spun at 1700 rpm at 5°C for 10 minutes. Supernatant was removed and the pellet resuspended in 15 mL CMF-HBSS; this step was repeated. The suspension was filtered through 0.05 g of glass wool in a 10 mL syringe. The effluent was spun again, the supernatant removed and the pellet was resuspended in 2 mL of RPMI-1640 (RPMI 1640 (Sigma, St. Louis, MO) 500 mL + 50000 U penicillin/50mg streptomycin) + 25 mL fetal bovine serum (Sigma, St. Louis, MO)).
Lamina propria analysis
After PP were removed the SI was threaded over a plastic catheter and opened longitudinally. It was cut into 1-cm pieces and placed in 50-mL tubes containing 25 mL of cold CMF-HBSS and incubated at room temperature for 15 minutes. The supernatant was removed and 25 mL EDTA/CMF-HBSS (10 mM (3.72 g) EDTA (Sigma, St. Louis, MO)/1000 mL of CMF-HBSS (as above)) pre-warmed at 37°C was added and incubated for 20 min with a magnetic stirrer at 100 rpm. The supernatant was removed and discarded. This step was repeated two more times. After the third incubation and removal, 25 mL HBSS was added to wash and the supernatant removed. Tissue was then incubated in 20 mL RPMI-1640 prewarmed at 37°C for 20 minutes on a magnetic stirrer at 100 rpm. Supernatant was discarded and 25 mL of RPMI-1640 with collagenases (500 mL RPMI-1640 + 5000 U collagenase type III (Sigma, St. Louis, MO) + 15000 U collagenase type I) added and incubated for 45 minutes on a magnetic stirrer at 100 rpm at 37°C. Supernatant was collected into 2 × 50-mL tubes. This step was repeated twice more and after the third incubation and collection 25 mL RPMI-1640 was added to wash and the supernatant was again collected. The collected supernatant from the 2 × 50-mL tubes was strained through 0.05 g glass wool in a 10-mL syringe and collected in new 50-mL tubes. Tubes were then centrifuged for 10 min at 1700 rpm at 5°C. Supernatant was discarded and Percoll 40% (diluted in RPMI-1640) (Pharmacia LKB, Uppsala, Sweden) was added, 2 mL to one tube and 3 mL to the second tube. These were then combined for a total of 5 mL and added slowly via a glass pipette to 4 mL of Percoll 75% in a 15-mL tube. We then centrifuged for 20 minutes at 1700 rpm and 5°C without brake. The top layer of debris was removed and the cell layer from the interface between the two Percoll concentrations was pipetted off with a glass pipette. This was transferred to a new 15-mL tube and the volume adjusted to 13 mL with addition of RPMI-1640. We then centrifuged at 1700 rpm for 10 minutes at 5°C. The supernatant was removed and the pellet was resuspended in 2 mL of RPMI-1640.
Spleen
The spleen was removed from one Chow-fed animal and placed in a Petri dish with 10 mL of cold RPMI. It was strained through a 40-μm nylon mesh with a 1-mL syringe plunger and the cell suspension was transferred to a 15-mL tube which was spun at 1700 rpm for 10 min at 5°C. The supernatant was discarded and the pellet was resuspended in 5 mL of 1X Lysis buffer (PharM Lyse, Becton-Dickinson, Franklin Lakes, NJ). This was vortexed and incubated for 15 min at room temperature protected from light. The tube was then spun at 1700 rpm for 10 min at 5°C. The supernatant was discarded and the pellet resuspended with 2 mL of cold RPMI. These cells were used for the compensation tubes for flow cytometry.
Surface Cell Marker Staining
Cells were counted by Trypan blue technique and the cell density was adjusted to approximately 1.0 × 106 cells/mL with staining buffer (Becton-Dickinson, Franklin Lakes, NJ). We then transferred 75 μL of the cell solution to a clean 5-mL tube (Falcon 2058) × 2 and added 10 μL of antibody solution (T cell antibody solution or B cell antibody solution). The T cell antibody solution contained the following fluorochrome conjugated antibodies from BD Pharmingen (San Diego, CA) at a final concentration of 2.5 μg/mL in staining buffer: Pacific Blue-conjugated mAb to CD3 (145-2C11), Alexa 700-conjugated mAb to CD4 (RM4-5), PerCP-Cy5.5-conjugated mAb to CD8a (53-6.7), APC-Cy7-conjugated mAb to L-selectin (MEL-14), APC-conjugated mAb to CD44 (IM7), PE-conjugated mAb to α4β7 (DATK32), and FITC-conjugated mAb to CD25 (7D4). The B cell antibody solution contained the following fluorochrome conjugated antibodies from BD Pharmingen at a final concentration of 2.5 μg/mL: Pacific Blue-conjugated mAb to CD3 (145-2C11), Alexa 700-conjugated mAb to CD45R/B220 (RA3-6B2), PerCP-Cy5.5-conjugated mAb to IgM (R6-60.2), APC-Cy7-conjugated mAb to L-slectin (MEL-14), APC-conjugated mAb to CD44 (IM7), PE-conjugated mAb to α4β7 (DATK32), and FITC-conjugated mAb to IgD (11-26c.2a). After addition of the antibody solution cells were incubated for 20 min on ice protected from light. We then added 1 mL of staining buffer, vortexed, and tubes were spun at 1000 rpm for 10 min at 5°C. The supernatant was then aspirated and 1 mL of staining buffer added to wash. This step was repeated and tubes were then stored at 4°C protected from light until flow cytometry was initiated.
Flow Cytometry Analysis
The BD LSR II Flow cell cytometer at the Wisconsin Institute of Medical Research was used for sample analysis. FlowJo flow cytometry analysis software (Ashland, OR) was used to analyze the raw data. We initially gated for lymphocytes, then B cells & T cells, and finally the various subsets. Percentages of cells of a given phenotype were calculated as a function of the parent cell population. A representative figure demonstrating our gating strategy is seen in figure 1. These percentages were compared using analysis of variance (ANOVA) and the Fisher protected least significance difference (PLSD) test, with α = 0.05 (Statview 5.0.1, SAS, Cary, NC). Numerical results are presented as mean ± standard error of the mean.
Figure 1. Representative gating strategy used for defining lymphocyte subset populations.
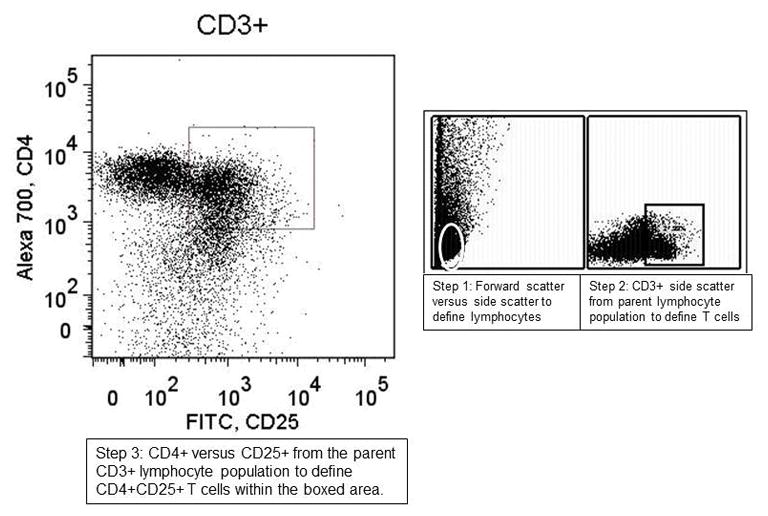
The gating strategy used involved first defining lymphocyte populations from the forward and side scatter and then defining subsequent sets from the preceding parent population based on positivity or negativity for our desired labels. Once in area was defined (as seen in the boxed areas on the figure) it was used for all samples to allow for comparison between the sample groups.
Experiment 2
Thirty-one mice were randomized to three diet groups (chow, n = 11; PN, n = 10, PN+BBS, n=10), anesthetized with an intraperitoneal ketamine (100 mg/kg) and acepromazine (5 mg/kg) mixture and cannulated via the right external jugular vein (0.012-in ID/0.25-in OD; Helix Medical, Inc, Carpinteria, CA). The mice underwent the same feeding and injections as the mice in experiment 1; however, LP lymphocytes were isolated and stained for expression of markers for Treg (CD4+CD25+Foxp3+) cells.
Intracellular staining for Foxp3
Cells were counted by Trypan blue technique and the cell density was adjusted to approximately 1.0 × 106 cells/mL with staining buffer (Becton-Dickinson, Franklin Lakes, NJ). We then transferred 75 μL of the cell solution to a clean 5-mL tube (Falcon 2058) × 2 and added 10 μL of antibody solution for extracellular staining as above. This surface marker panel contained the following fluorochrome conjugated antibodies from BD Pharmingen (San Diego, CA) at a final concentration of 2.5 μg/mL in staining buffer: APC conjugated mAb to CD4 (L3T4), PerCP-Cy5.5-conjugated mAb to CD25 (PC61.5), PE-conjugated mAb to CD3 (145-2C11). Before staining with FITC-conjugated mAb to Foxp3 (FJK-16s), the cells were fixed and permeabilized with Cytofix/Ctyoperm (BD Pharmigen, San Diego, CA) at 4° C for 30 minutes. Staining and washing were performed in Perm/Wash buffer (BD Pharmigen) for 45 minutes, and cells were washed once in and re-suspended in stain buffer (BD pharmigen) prior to analysis.
RESULTS
Experiment 1
T-cell Subsets
Experiment 1: Within the lamina propria (Figure 2), PN significantly decreased CD4+CD25+ (activated) T cells compared to Chow (PN: 24% ± 4.4 vs Chow: 43% ± 3.8, p = 0.01) while PN+BBS significantly increased the CD4+CD25+ cells compared to PN (PN+BBS: 39% ± 4.4 vs PN: 24% ± 4.4, p < 0.05). There was no effect of PN or BBS on CD4+CD25+cells within the Peyer’s patches. Within the lamina propria (Figure 3), CD4+CD25+LPAM-1+ cells (activated cells targeted to the lamina propria effector site) were significantly reduced in PN compared to Chow (PN: 12% ± 2.3 vs Chow 25% ± 1.9, p < 0.05) while the addition of BBS to PN significantly increased CD4+CD25+LPAM-1+ cells in the LP compared to PN back to Chow levels (PN+BBS: 27% ± 2.4 vs. PN: 12% ± 2.3, p < 0.05). No significant changes were seen in CD4+L-selectin+ or CD4+CD44+ T cells in the LP (Table 3). Also, there were no significant changes in PP of any CD4+ T cell phenotypes (Table 3). There were no significant differences occurred in any CD8+ T cell populations in the PP or LP (data not shown).
Figure 2. CD4+CD25+ lymphocytes from lamina propria and Peyer’s Patches.
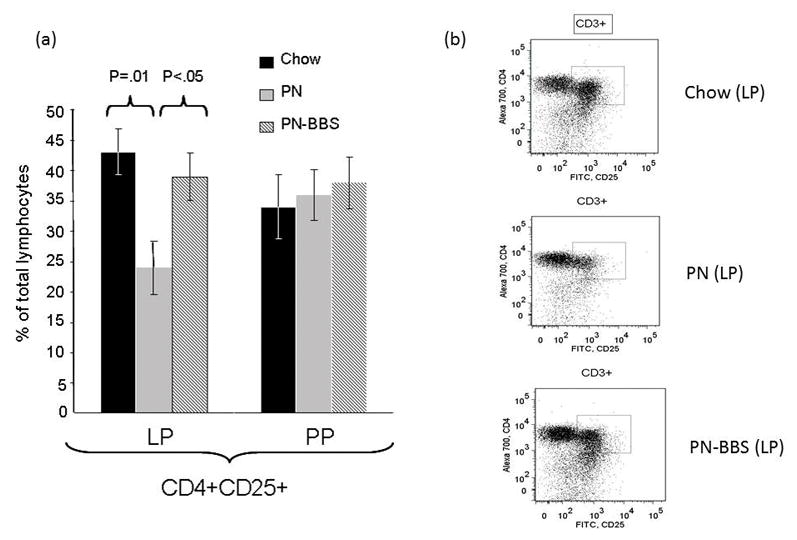
(a)Parenteral nutrition (PN) significantly suppresses the percentage of cells expressing CD4+CD25+ compared to Chow (24% ± 4.4 vs. 43% ± 3.8, p = 0.01). There were no changes in this population in the Peyer’s Patches. Addition of bombesin (BBS) to PN (PN+BBS) significantly increased CD4+CD25+ lymphocytes in the lamina propria compared to PN alone (39% ± 4.4 vs. 24% ± 4.4, p < 0.05) while no changes were seen in the Peyer’s Patches. Values are means ± SE. Flow cytometry gating progressed from the preceding parent population in the order of lymphocytes, CD3+, and then CD4+CD25+ as seen in figure 1. (b)Representative flow cytometry figures from Chow, PN, and PN+BBS demonstrating CD3+ T cells from the parent lymphocyte population. The CD3+ cells were then plotted as CD4+ versus CD25+ and an area (seen in plots as box) of positivity for both markers was determined that was then used for all samples.
Figure 3. CD4+CD25+α4β7+ lymphocytes from lamina propria.
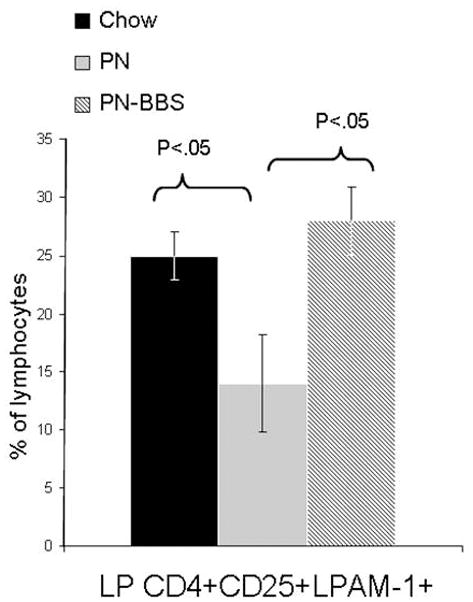
Parenteral nutrition (PN) significantly reduces the percentage of cells expressing CD4+CD25+ α4β7+ compared to chow (12% ± 2.3 vs. 25% ± 1.9 vs. , p < 0.05). Addition of bombesin (BBS) to PN (PN+BBS) significantly increased CD4+CD25+ α4β7+ lymphocytes in the lamina propria compared to PN (27% ± 2.4 vs. 12% ± 2.3, p < 0.05). Values are means ± SE. Flow cytometry gating progressed from the preceding parent population in the order of lymphocytes, CD3+, CD4+CD25+, and then α4β7+ cells.
Table 3.
T cell phenotypes without significant changes between Chow, PN, and PN-BBS
| Lymphocyte Populations | |||
| PP T cells | Chow | PN | PN-BBS |
| CD4+CD25+Foxp3+ | 34.1 ± 5.2 % | 36.2 ± 4.2 % | 38.3 ± 4.3 % |
| CD4+CD25+LPAM-1+ | 8.8 ± 1.1 % | 11.1 ± 1.5 % | 9.3 ± 1.4 % |
| CD4+L-selectin+ | 43.2 ± 4.2 % | 35.6 ± 2.8 % | 40.4 ± 1.4 % |
| CD4+CD44+ | 56.9 ± 3.9 % | 60.7 ± 2.1 % | 61.6 ± 1.6 % |
| LP T cells | Chow | PN | PN-BBS |
| CD4+L-selectin+ | 56.2 ± 3.9 % | 51.9 ± 3.4 % | 59.1 ± 3.9 % |
| CD4+CD44+ | 59.6 ± 4.4 % | 60.4 ± 6.5 % | 60.2 ± 7.5 % |
Data expressed as percentage of the parent cell population (CD4+ T cells)
Abbreviations: PP (Peyer’s patches), LP (lamina propria), PN (parenteral nutrition), PN-BBS (parenteral nutrition with bombesin)
B-cell Subsets
PN significantly reduced three B cell populations in the LP. Firstly (Figure 4), IgD+ (unactivated naïve) cells decreased significantly in the LP with PN compared to Chow (PN: 23% ± 2.5 vs Chow: 34% ± 1.8, p < 0.05). PN+BBS (30% ± 2.4) reversed this decrease significantly increasing the IgD+ cells in the LP compared to PN (PN+BBS: 30% ± 2.4 vs PN: 23% ± 2.5, p < 0.05). Secondly (Figure 4), PN significantly decreased IgD- LPAM-1+ (antigen stimulated B cells expressing for homing to the lamina propria) cells in the LP compared to Chow (PN: 2% ± 0.6 vs. Chow: 6% ± 1.2, p <0.05) while PN+BBS (5% ± 1.1) significantly increased this population from PN levels (PN+BBS: 5% ± 1.12% vs PN: 2% ± 0.6, p < 0.05) back to Chow levels. Finally (Figure 4), PN significantly reduced CD44+ (memory) B cells compared to Chow (PN: 21% ± 6.4 vs Chow: 38% ± 4.0, p < 0.05) while BBS significantly increased the CD44+ B cell population from PN values (PN+BBS: 33% ± 4.3 vs. PN: 21% ± 4.2, p < 0.05). There were no significant differences between groups of animals regarding B cell populations in the PP and no changes in IgM+ B cells including those with L-selectin or α4β7 expression (Table 4).
Figure 4. B cell subsets of lymphocytes from lamina propria.
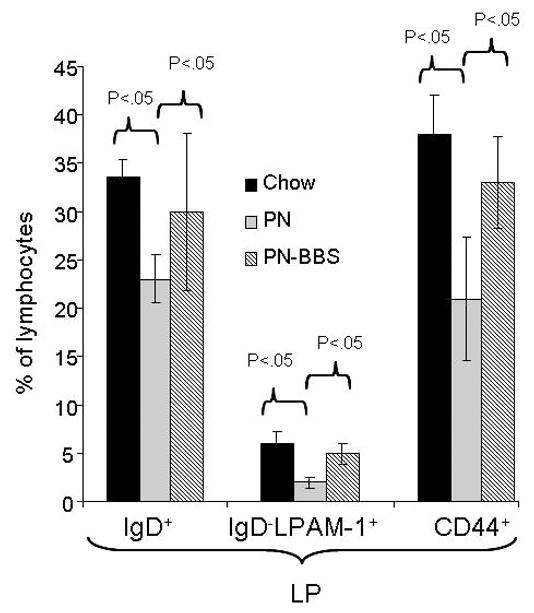
Parenteral nutrition (PN) significantly reduces IgD+(23% ± 2.5 vs. 34% ± 1.8, p <0.05), IgD- α4β7+(2% ± 0.6 vs. 6% ± 1.2, p<0.05), and CD44+(21% ± 4.2 vs. 38% ± 4.0, p<0.05) B cells in the lamina propria compared to chow. Addition of bombesin (BBS) to PN (PN+BBS) significantly increased IgD+(30% ± 2.4 vs. 23% ± 2.5, p<0.05), IgD- α4β7+(5% ± 1.1 vs. 2% ± 0.6, p<0.05), CD44+(33% ± 4.3 vs. 21% ± 4.2, p<0.05) B lymphocytes in the lamina propria. Values are means ± SE. Flow cytometry gating progressed from the preceding parent population in the order of lymphocytes, CD45/B220, and then IgD+ or IgD- α4β7+ or CD44+ subsets respectively.
Table 4.
B cell phenotypes without significant changes between Chow, PN, and PN-BBS
| Lymphocyte Populations | |||
| PP B cells | Chow | PN | PN-BBS |
| IgD+ | 19.7 ± 5.6 % | 19.7 ± 7.3 % | 23.2 ± 8.2 % |
| CD44+ | 67.6 ± 3.4 % | 58.5 ± 7.2 % | 56.1 ± 9.8 % |
| IgM+ | 4.2 ± 0.8 % | 4.7 ± 0.3 % | 5.1 ± 0.8 % |
| IgM+L-selectin+ | 46.1 ± 2.7 % | 42.8 ± 1.3 % | 35.6 ± 4.2 % |
| IgM+LPAM-1+ | 53.5 ± 8.6 % | 48.1 ± 1.4 % | 54.1 ± 6.7 % |
| LP B cells | Chow | PN | PN-BBS |
| IgM+ | 11.2 ± 1.8 % | 9.7 ± 0.9 % | 9.8 ± 0.2 % |
| IgM+L-selectin+ | 40.2 ± 1.9 % | 36.2 ± 2.6 % | 38.2 ± 1.5 % |
| IgM+LPAM-1+ | 35.6 ± 3.1 % | 36.4 ± 2.1 % | 36.3 ± 3.1 % |
Data expressed as percentage of the parent cell population (B cells)
Abbreviations: PP (Peyer’s patches), LP (lamina propria), PN (parenteral nutrition), PN-BBS (parenteral nutrition with bombesin)
Experiment 2
To investigate the phenotypic changes in the lamina propria CD4+CD25+ subpopulations noted in experiment 1, these cells were examined for expression of CD4+CD25+Foxp3+ to measure alterations in the Treg population (Figure 5). PN significantly decreased Tregs cells compared to Chow (PN: 38% ± 2.2 vs Chow: 58% ± 1.9, p =0.01) while PN+BBS significantly increased the Treg cells from PN values (BBS+PN: 59% ± 2.3 vs PN: 38% ± 2.2, p = 0.01) to Chow levels.
Figure 5. CD4+CD25+Foxp3+ lymphocytes from lamina propria.
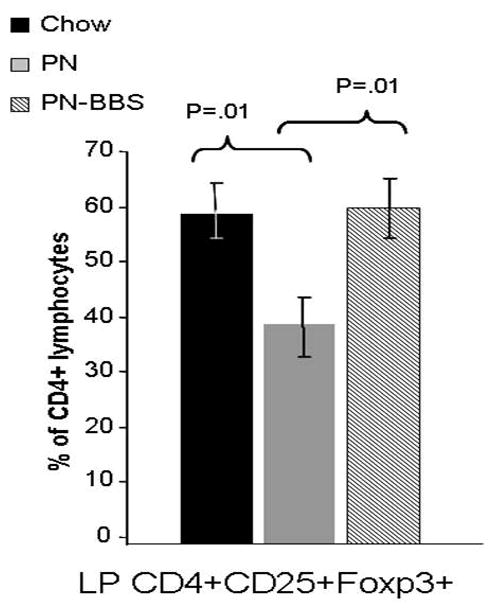
Parenteral nutrition (PN) significantly reduces the percentage of cells expressing CD4+CD25+Foxp3+ compared to chow (38% ± 2.2 vs. 58% ± 1.9, p =0.01). Addition of bombesin (BBS) to PN (PN+BBS) significantly increased CD4+CD25+Foxp3+ lymphocytes in the lamina propria compared to PN (59% ± 2.3 vs. 38% ± 2.2, p =0.01). Values are means ± SE. Flow cytometry gating progressed from the preceding parent population in the order of lymphocytes, CD3+, CD4+CD25+, and finally Foxp3+.
DISCUSSION
Parenteral feeding with lack of enteral stimulation increases the incidence of infectious complications in critically injured trauma patients and other critically ill general surgery patients.2–5 While multiple factors influence this vulnerability, alterations in mucosal immunity provide a cogent explanation for these observations. Experimentally, PN with decreased enteral nutrition (PN/DES) compromises the mucosal immune system of mice by down-regulating expression of the adhesion molecules, chemokines, and transport molecules critical to the entry and distribution of B & T cells throughout the common mucosal immune system. 6–8 PN also impairs the production and transport of secretory IgA into both intestinal and pulmonary secretions by reducing tissues levels of two important Th-2 type IgA-stimulating cytokines, IL-4 and IL-10 as well as the transport protein pIgR. 27 IgA functions in immune exclusion through binding to the specific antigens on the surface of luminal pathogens preventing their ability to attach to mucosal surfaces and thus precluding tissue invasion. Functionally, these PN-induced defects result in loss of established IgA-mediated immunity against both viral and bacterial pathogens. 14, 28 Experimentally, exogenous administration of neuropeptides produced and released by the enteric nervous system (ENS) prevents these PN-induced anti-viral and anti-bacterial defects in IgA-mediated mucosal defenses. 13, 21–23 BBS, an analogue of gastrin-releasing peptide, represents one of the first neuropeptides released in response to enteral feeding.
In addition to bacterial exclusion 29, 30, IgA also plays a role in controlling inflammatory responses 31, 32 and IgA binding to luminal antigen facilitates controlled entry of luminal antigens into the principal inductive site, the Peyer’s patches, via binding of IgA-antigen complexes to M cells which cover the luminal surface of this site. The IgA-antigen complex binds to dendritic cells for processing and either an inflammatory or anti-inflammatory response results depending on multiple co-stimulatory signals. Production of IgA in the lamina propria (LP) results after sensitization and activation of previously naïve T & B lymphocytes in the Peyer’s patches. 33, 34 These sensitized T & B cells are released into lymphatics, migrate into mesenteric lymph nodes (where they mature and/or proliferate), proceed through the thoracic duct into the blood stream and home to effector sites in the LP where CD4+ T cells stimulate IgA production via an interaction with activated B cells (after transformation into plasma cells) through Th-2 type IgA-stimulating cytokines (IL-4, IL-5, IL-6, IL-10).5, 27 The integrin α4β7 (measured by LPAM-1 in these experiments) directs these activated cells into the appropriate effector lamina propria site. In prior work we noted that PN results in 50–60% reductions of T & B lymphocytes within the Peyer’s patches and lamina propria of the lung and small intestine. Enteral feeding maintains expression of the critical gateway molecule, mucosal addressin cellular adhesion molecule -1 (MAdCAM-1), expressed on the high endothelial venules of the Peyer’s patches. MAdCAM-1 mediates naïve T and B cell entry into the PP via interaction with the L-selectin integrin (measured by CD62L in these experiments) expressed on the lymphocytes and the presence of site-specific chemokines present in the region. MAdCAM-1 levels drop rapidly during PN and recover within hours of reinstituting enteral feeding. Cause and effect relationships between MAdCAM-1 and L-selectin was established experimentally by blockade of MAdCAM-1 or L-selectin with a specific monoclonal antibodies during chow feeding: GALT lymphocytes numbers dropped to the levels of PN fed mice following blockade of ether molecule. 35 Experimentally we documented that administration of BBS during PN not only reverses the negative effects of PN/DES on IgA levels and maintained normal lymphocyte numbers in GALT, but it also maintains established IgA-mediated immunity against both viral and bacterial challenges. Initially, we suspected that BBS worked by preserving MAdCAM-1 expression. However, subsequent work clearly showed that the depressed levels of MAdCAM-1 remained unaffected by BBS administration, i.e. the mechanism of BBS was not due to maintenance of T & B cell entry into GALT through this molecule. The current work demonstrates that BBS maintains IgA levels by preserving levels of specific T & B GALT lymphocyte phenotypes to maintain mucosal immune integrity.
In this work, we examined changes in phenotypic markers of activation (CD25), memory (CD44) homing (L-selectin: homing to Peyer’s patches and α4β7: homing to lamina propria), T regulatory cells (Foxp3), and naïve B cells (IgD) in addition to the B, T helper, CD4 and CD8 populations defined in our prior work. Our current results document a paucity of population shift changes in cells types within the Peyer’s patches; here the T helper distribution remained unaffected by PN with or without BBS. These results confirm our prior work demonstrating a constant CD4/CD8 ratio within this compartment despite reductions in absolute cell numbers in the Peyer’s patches with PN. However within the lamina propria, where IgA is produced for specific mucosal immune defense, PN/DES induced significant phenotypic changes in both T and B cell populations. PN/DES altered the CD4+ T cell phenotype in the LP by decreasing the activated T cell phenotype (CD4+CD25+) and most notably the Treg phenotype (CD4+CD25+Foxp3+). In addition, PN/DES decreased the percentage of activated T cells (CD4+CD25+) expressing the homing molecule α4β7 (LPAM-1) which directs cells into the lamina propria. Not surprisingly, T cell expression of L-selectin (CD62) was not affected since L-selectin is expressed on naïve lymphocytes and is responsible for migration into Peyer’s patches while expression of α4β7 on activated lymphocytes mediates entry into effector sites (i.e. the lamina propria) in concert with various chemokine receptors 36. Therefore, PN/DES does not appear to affect naïve lymphocytes at these locations, particularly within the PP where naïve lymphocytes become sensitized after entry. Rather, PN/DES reduces antigen-activated T cells expressing α4β7 which must subsequently migrate to the effector sites to repopulate IgA producing lymphocytes. One specific phenotype of interest is the lamina propria CD4+CD25+Foxp3+ T regulatory cell (Treg) population which were reduced with PN but normalized with BBS. Recognized within the last 15 years, Tregs provide antigen-specific stimulatory signals via TGFβ and IL-10 to increase IgA+ B cell IgA production while simultaneously suppressing an IgA-inhibiting cytokine, IFNγ within the LP.37 Tregs play an important role in maintaining immunological self-tolerance by suppressing potentially autoreactive T cells. Addition of BBS reverses these changes restoring all of these cell populations to chow levels. Thus, BBS maintains mucosal immunity primarily through influencing cell populations within the LP effector site.
PN/DES also affected B cells subpopulations within the lamina propria resulting in reductions in IgD+, IgD- LPAM+, and CD44+ memory cells, each of which normalized with BBS administration. While T cells control IgA production, IgA synthesis depends upon plasma cells transformed from activated/sensitized B cells. Lamina propria IgD+ cells represent mature B cells capable of receiving antigen presentation and stimulation 38. In contrast to IgM+ B cells, IgD+ B cells concentrate at mucosal surfaces and respond to T cells for activation 39. After activation by antigen presentation and T cell stimulation, IgD+ B cells lose IgD expression and undergo class switching to a specific immunoglobulin class such as IgA. 38–40 Additionally, activation results in integrin expression changes with increases in α4β7 (represented by being LPAM+) that target the activated B cells to effector sites. In our model, PN+BBS increased cells expressing the phenotype, IgD- α4β7+, in the LP compared to PN feeding alone. These B cell changes depict increases in activated B cells at effector sites, capable of promoting IgA production. Finally, PN+BBS restored B cells CD44+ expression to Chow levels. CD44 is a glycoprotein adhesion molecule that facilitates lymphocyte interaction with endothelium and functions as a marker of a memory or effector cell. 41 B cells with increased CD44 expression reflect antigen-specific immunoglobulin producing cells that have undergone class switching in response to prior antigen challenge. 42 Finally, no changes in B cell phenotypes were observed in the PP, indicating that although PN reduces B cell overall numbers at these inductive sites, the proportionate cell types present are not altered. Overall, BBS increases B cells in the LP which can respond to antigen (IgD+), increases cells which are antigen activated and home to effector sites (IgD- α4β7+), and increases the cells capable of immunoglobulin production and are antigen specific (CD44+) compared to cells in LP of PN mice.
Unfortunately, this work fails to explain how GALT cell numbers are preserved during PN and especially how BBS treatment returned GALT cells numbers from their nadir to normal when administered to mice on their 5th, 6th and 7th day of PN. 21 Reduced apoptosis prolonging the lifespan of lymphocytes could explain maintenance of normal numbers if BBS is initiated immediately; this phenomenon has been documented to occur in intestinal cells during glutamine administration. It would not explain how the addition of BBS leads to recovery of lymphocytes from depressed levels. Does BBS allow an alternative method for replenishing lamina propria cells which is not dependent on MAdCAM-1 entry of naïve lymphocytes into GALT? Miyasaka et al also recently showed a significant reduction in LP Tregs with PN/DES versus enteral feeding and additionally no change in LP Tregs with PN/DES in mice lacking a component of the pro-inflammatory NF-κB signaling pathway (MyD88 knockout mice). 43 Could BBS effect this signaling pathway and subsequently LP Treg populations? These questions require further investigation.
In summary, the majority of changes in T & B cell phenotypes with parenteral feeding occur at effector sites with no shifts in cell populations noted in the Peyer’s patches, the main inductive sites for mucosal immune responses. Neuropeptide administration prevents these alterations. Reductions in Tregs at the mucosal effector sites fit mechanistically with the role of Tregs in stimulating IgA+ B cells and IgA production and are consistent with the PN-induced decreases in the three B cell populations seen in the LP. The B cells rely on signals from Tregs for activation necessary to produce and secrete IgA. The mechanism through with the neuropeptide, BBS, preserves mucosal IgA-mediated immunity appears to be through a cellular effects on specific LP lymphocyte populations that are involved in IgA production.
Acknowledgments
Supported by:
This research is supported by National Institute of Health (NIH) Grant R01 GM53439. This material is also based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development Service. The contents of this article do not represent the views of the Dept. of Veterans Affairs or the United States Government.
Footnotes
The work was originally presented February 5, 2010 at the Association for Academic Surgery meeting in San Antonio, TX
Conflicts of Interest and Source of Funding:
There are no conflicts of interest.
References
- 1.Heyland DK, Cook DJ, Griffith L, et al. The attributable morbidity and mortality of ventilator-associated pneumonia in the critically ill patient. The Canadian Critical Trials Group. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1249–56. doi: 10.1164/ajrccm.159.4.9807050. [DOI] [PubMed] [Google Scholar]
- 2.Moore EE, Jones TN. Benefits of immediate jejunostomy feeding after major abdominal trauma--a prospective, randomized study. J Trauma. 1986;26(10):874–81. doi: 10.1097/00005373-198610000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Moore F, Moore E, Jones T, et al. TEN versus TPN following major abdominal trauma--reduced septic morbidity. J Trauma. 1989;29(7):916–22. doi: 10.1097/00005373-198907000-00003. discussion 922–3. [DOI] [PubMed] [Google Scholar]
- 4.Moore FA, Feliciano DV, Andrassy RJ, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg. 1992;216(2):172–83. doi: 10.1097/00000658-199208000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kudsk K, Croce M, Fabian T, et al. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992;215(5):503–11. doi: 10.1097/00000658-199205000-00013. discussion 511–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Kudsk K, Gocinski B, et al. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma. 1995;39(1):44–51. doi: 10.1097/00005373-199507000-00006. discussion 51–2. [DOI] [PubMed] [Google Scholar]
- 7.King B, Li J, Kudsk K. A temporal study of TPN-induced changes in gut-associated lymphoid tissue and mucosal immunity. Arch Surg. 1997;132(12):1303–9. doi: 10.1001/archsurg.1997.01430360049009. [DOI] [PubMed] [Google Scholar]
- 8.Fukatsu K, Kudsk K, Zarzaur B, et al. TPN decreases IL-4 and IL-10 mRNA expression in lipopolysaccharide stimulated intestinal lamina propria cells but glutamine supplementation preserves the expression. Shock. 2001;15(4):318–22. doi: 10.1097/00024382-200115040-00012. [DOI] [PubMed] [Google Scholar]
- 9.Hermsen J, Gomez F, Sano Y, et al. Parenteral feeding depletes pulmonary lyphocyte populations. J Parenter Enteral Nutr. 2009;33(5):535–40. doi: 10.1177/0148607109332909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sano Y, Hermsen J, Kang W, et al. Parenteral nutrition maintains pulmonary IgA antibody transport capacity, but not active transport, following injury. Am J Surg. 2009 doi: 10.1016/j.amjsurg.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson C, Kudsk K, Fukatsu K, et al. Route of nutrition influences generation of antibody-forming cells and initial defense to an active viral infection in the upper respiratory tract. Ann Surg. 2003;237(4):565–73. doi: 10.1097/01.SLA.0000059991.89316.B8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y, Kudsk K, DeWitt R, et al. Route and type of nutrition influence IgA-mediating intestinal cytokines. Ann Surg. 1999;229(5):662–7. doi: 10.1097/00000658-199905000-00008. discussion 667–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janu PG, Kudsk KA, Li J, et al. Effect of bombesin on impairment of upper respiratory tract immunity induced by total parenteral nutrition. Arch Surg. 1997;132(1):89–93. doi: 10.1001/archsurg.1997.01430250091019. [DOI] [PubMed] [Google Scholar]
- 14.King BK, Kudsk KA, Li J, et al. Route and type of nutrition influence mucosal immunity to bacterial pneumonia. Ann Surg. 1999;229(2):272–8. doi: 10.1097/00000658-199902000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kudsk KA, Hermsen JL, Genton L, et al. Injury stimulates an innate respiratory immunoglobulin a immune response in humans. J Trauma. 2008;64(2):316–23. doi: 10.1097/TA.0b013e3181627586. discussion 323–5. [DOI] [PubMed] [Google Scholar]
- 16.Ottaway CA. Neuroimmunomodulation in the intestinal mucosa. Gastroenterol Clin North Am. 1991;20(3):511–29. [PubMed] [Google Scholar]
- 17.Ghatei MA, Jung RT, Stevenson JC, et al. Bombesin: action on gut hormones and calcium in man. J Clin Endocrinol Metab. 1982;54(5):980–5. doi: 10.1210/jcem-54-5-980. [DOI] [PubMed] [Google Scholar]
- 18.Miyata M, Rayford PL, Thompson JC. Hormonal (gastrin, secretin, cholecystokinin) and secretory effects of bombesin and duodenal acidification in dogs. Surgery. 1980;87(2):209–15. [PubMed] [Google Scholar]
- 19.Bienenstock J, Perdue M, Stanisz A, et al. Neurohormonal regulation of gastrointestinal immunity. Gastroenterology. 1987;93(6):1431–4. doi: 10.1016/0016-5085(87)90277-0. [DOI] [PubMed] [Google Scholar]
- 20.Jin GF, Guo YS, Houston CW. Bombesin: an activator of specific Aeromonas antibody secretion in rat intestine. Dig Dis Sci. 1989;34(11):1708–12. doi: 10.1007/BF01540048. [DOI] [PubMed] [Google Scholar]
- 21.DeWitt RC, Wu Y, Renegar KB, et al. Bombesin recovers gut-associated lymphoid tissue and preserves immunity to bacterial pneumonia in mice receiving total parenteral nutrition. Ann Surg. 2000;231(1):1–8. doi: 10.1097/00000658-200001000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Kudsk KA, Hamidian M, et al. Bombesin affects mucosal immunity and gut-associated lymphoid tissue in intravenously fed mice. Arch Surg. 1995;130(11):1164–9. doi: 10.1001/archsurg.1995.01430110022005. discussion 1169–70. [DOI] [PubMed] [Google Scholar]
- 23.Zarzaur BL, Wu Y, Fukatsu K, et al. The neuropeptide bombesin improves IgA-mediated mucosal immunity with preservation of gut interleukin-4 in total parenteral nutrition-fed mice. Surgery. 2002;131(1):59–65. doi: 10.1067/msy.2002.118319. [DOI] [PubMed] [Google Scholar]
- 24.Sitren HS, Heller PA, Bailey LB, et al. Total parenteral nutrition in the mouse: development of a technique. JPEN J Parenter Enteral Nutr. 1983;7(6):582–6. doi: 10.1177/0148607183007006582. [DOI] [PubMed] [Google Scholar]
- 25.Rogers TD, Gades NM, Kearby JD, et al. Chronic restraint via tail immobilization of mice: effects on corticosterone levels and other physiologic indices of stress. Contemp Top Lab Anim Sci. 2002;41(1):46–50. [PubMed] [Google Scholar]
- 26.Nutrition CoA. Nutrient Requirements of Laboratory Animals. Washington D.C: National Academy Press; 1995. Vol. Fourth Revised Edition. [Google Scholar]
- 27.Sano Y, Gomez F, Hermsen J, et al. Parenteral nutrition induces organ specific alterations in polymeric immunoglobulin receptor levels. J Surg Res. 2008;149(2):236–42. doi: 10.1016/j.jss.2007.12.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kudsk K. Current aspects of mucosal immunology and its influence by nutrition. Am J Surg. 2002;183(4):390–8. doi: 10.1016/s0002-9610(02)00821-8. [DOI] [PubMed] [Google Scholar]
- 29.Albanese C, Smith S, Watkins S, et al. Effect of secretory IgA on transepithelial passage of bacteria across the intact ileum in vitro. J Am Coll Surg. 1994;179(6):679–88. [PubMed] [Google Scholar]
- 30.Niederman MS, Merrill WW, Polomski LM, et al. Influence of sputum IgA and elastase on tracheal cell bacterial adherence. Am Rev Respir Dis. 1986;133(2):255–60. doi: 10.1164/arrd.1986.133.2.255. [DOI] [PubMed] [Google Scholar]
- 31.Nizet V. Understanding how leading bacterial pathogens subvert innate immunity to reveal novel therapeutic targets. J Allergy Clin Immunol. 2007;120(1):13–22. doi: 10.1016/j.jaci.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Diebel L, Liberati D, Diglio C, et al. Immunoglobulin a modulates inflammatory responses in an in vitro model of pneumonia. J Trauma. 2005;59(5):1099–106. doi: 10.1097/01.ta.0000187797.38327.78. [DOI] [PubMed] [Google Scholar]
- 33.Brandtzaeg P. Mucosal immunity: induction, dissemination, and effector functions. Scand J Immunol. 2009;70(6):505–15. doi: 10.1111/j.1365-3083.2009.02319.x. [DOI] [PubMed] [Google Scholar]
- 34.Norderhaug IN, Johansen FE, Schjerven H, et al. Regulation of the formation and external transport of secretory immunoglobulins. Crit Rev Immunol. 1999;19(5–6):481–508. [PubMed] [Google Scholar]
- 35.Genton L, Kudsk K, Reese S, et al. Enteral feeding preserves gut Th-2 cytokines despite mucosal cellular adhesion molecule-1 blockade. JPEN J Parenter Enteral Nutr. 29(1):44–7. doi: 10.1177/014860710502900144. [DOI] [PubMed] [Google Scholar]
- 36.Hermsen J, Gomez F, Maeshima Y, et al. Decreased enteral stimulation alters mucosal immune chemokines. JPEN J Parenter Enteral Nutr. 2008;32(1):36–44. doi: 10.1177/014860710803200136. [DOI] [PubMed] [Google Scholar]
- 37.Feng T, Elson CO, Cong Y. Treg cell-IgA axis in maintenance of host immune homeostasis with microbiota. Int Immunopharmacol. 2011;11(5):589–92. doi: 10.1016/j.intimp.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strober W. Regulation of IgA B-cell development in the mucosal immune system. J Clin Immunol. 1990;10(6 Suppl):56S–61S. doi: 10.1007/BF00918692. discussion 61S–63S. [DOI] [PubMed] [Google Scholar]
- 39.Strober W, Harriman GR, Kunimoto DR. Early steps of IgA B cell differentiation. Immunol Res. 1991;10(3–4):386–8. doi: 10.1007/BF02919726. [DOI] [PubMed] [Google Scholar]
- 40.Strober W, Harriman GR. The regulation of IgA B-cell differentiation. Gastroenterol Clin North Am. 1991;20(3):473–94. [PubMed] [Google Scholar]
- 41.Haynes BF, Telen MJ, Hale LP, et al. CD44--a molecule involved in leukocyte adherence and T-cell activation. Immunol Today. 1989;10(12):423–8. doi: 10.1016/0167-5699(89)90040-6. [DOI] [PubMed] [Google Scholar]
- 42.Vitetta ES, Berton MT, Burger C, et al. Memory B and T cells. Annu Rev Immunol. 1991;9:193–217. doi: 10.1146/annurev.iy.09.040191.001205. [DOI] [PubMed] [Google Scholar]
- 43.Miyasaka EA, Feng Y, Poroyko V, et al. Total parenteral nutrition-associated lamina propria inflammation in mice is mediated by a MyD88-dependent mechanism. J Immunol. 2013;190(12):6607–15. doi: 10.4049/jimmunol.1201746. [DOI] [PMC free article] [PubMed] [Google Scholar]


