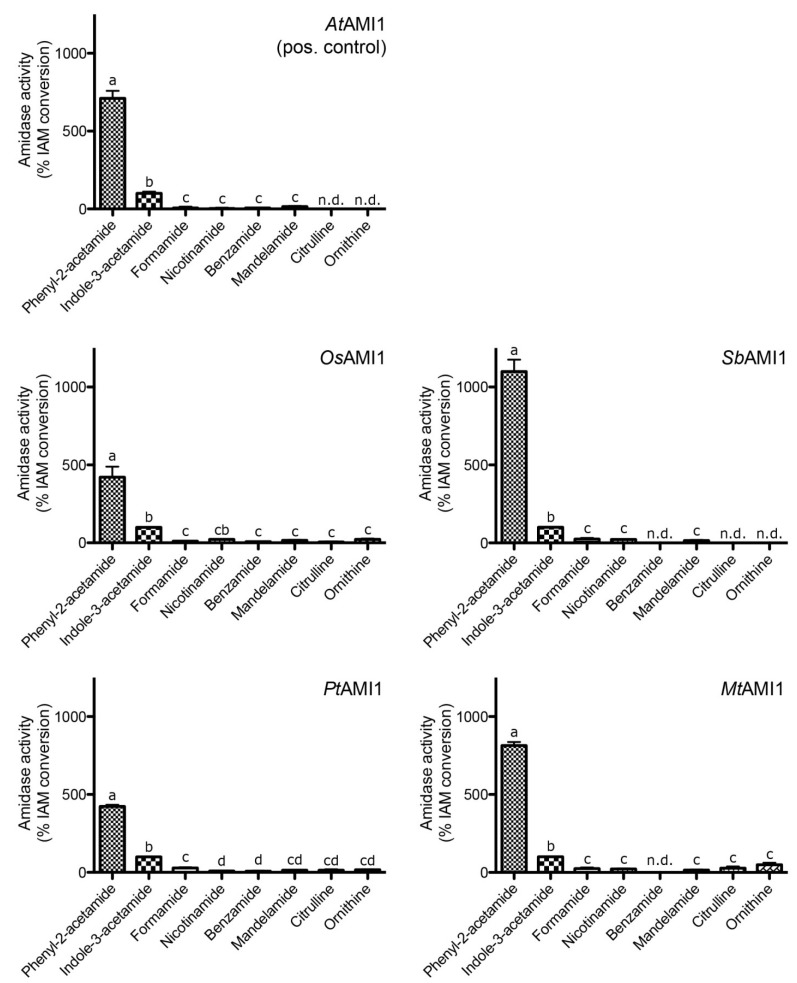
Figure 5.
Analysis of the relative substrate conversion of recombinant amidases.
All assays were performed in a total reaction volume of 300 μL at pH 7.5 and 30 °C (substrate concentration 10 mM, reaction time 4 to 6 h). All activities were normalized to the conversion of IAM (100%). Catalytic activities are corrected for background activities obtained for the non-enzymatic breakdown of substrates in heat-inactivated and empty-vector control samples. Maximum specific activities for IAM were 3070 pkat (mg protein)−1 (AtAMI1), 2378 pkat (mg protein)−1 (SbAMI1), 375 pkat (mg protein)−1 (OsAMI1), 37 pkat (mg protein)−1 (MtAMI1), and 329 pkat (mg protein)−1 (PtAMI1). The data shown are means ± SE derived from n ≥ 3 experiments. n.d.: not detected. Mean values within a graph are significantly different (p < 0.05) where superscript letters differ.