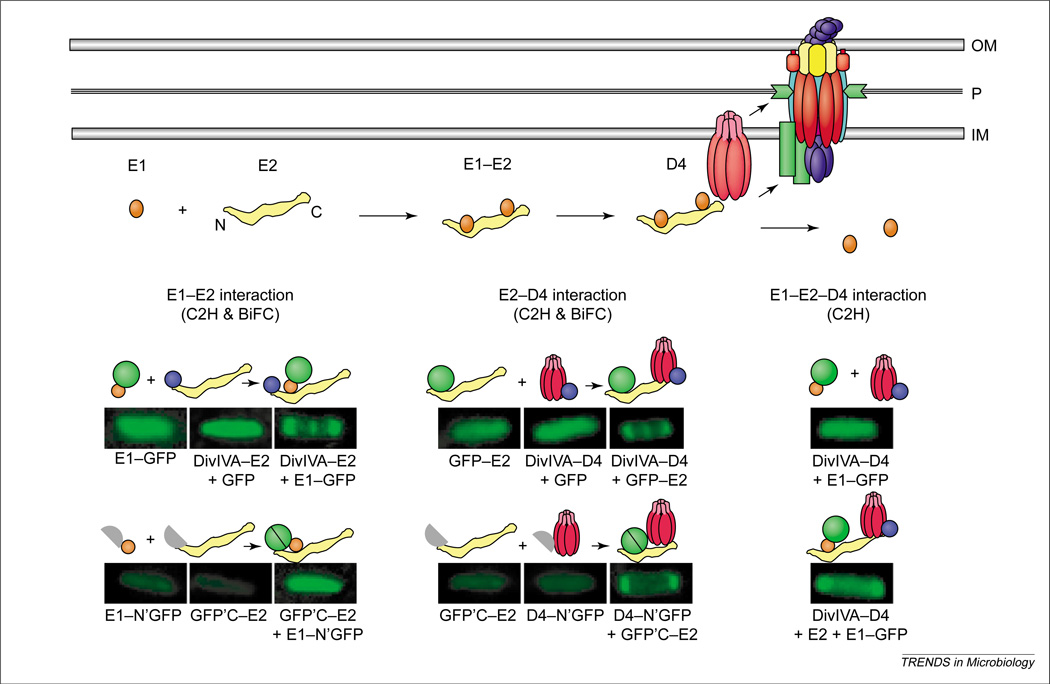
Figure 3.
Processing and recruitment of the VirE2 effector to the Agrobacterium tumefaciens VirB–D4 system. Top: Newly synthesized VirE2 forms a stabilizing complex with the secretion chaperone VirE1. Chaperone interactions with N-terminal and central domains prevent VirE2 self-aggregation and formation of premature complexes with other exported effectors, for example, the T-strand. A secretion signal located near the C-terminus of VirE2 mediates complex formation with the VirD4 T4CP (type IV coupling protein) without contributions from VirE1 or the VirB proteins. Lower: Genetic requirements for complex formation between VirE1 and VirE2, and between VirE2 and VirD4, as defined with novel cytology two-hybrid (C2H) and bimolecular fluorescence complementation (BiFC) assays. Fluorescence patterns of cells shown result from production of full- or half-length GFP fusion proteins listed below each image, and the patterns supply evidence for the interactions depicted above each image. Green circle, GFP; purple circle, DivIVA.