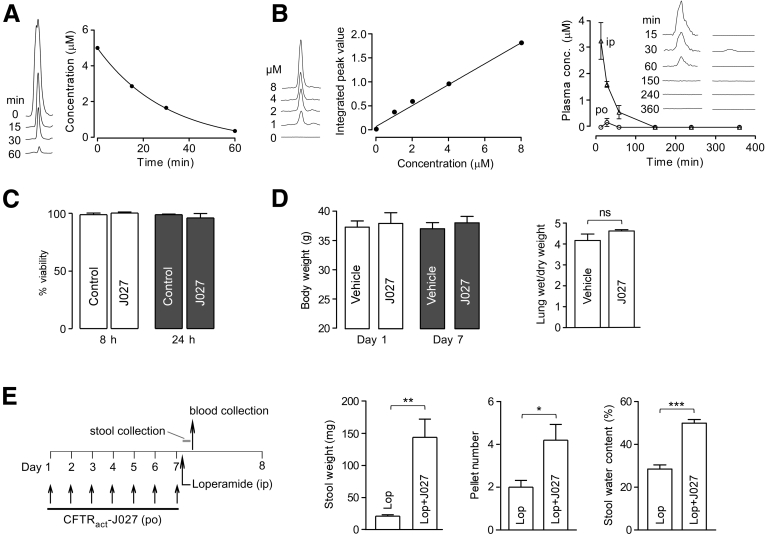
Figure 6.
CFTRact-J027 pharmacokinetics, tissue distribution, and toxicity. (A) In vitro metabolic stability of CFTRact-J027 assayed in mouse liver microsomes after incubation for specified times. (B) Standard plasma concentration curve for liquid chromatography–mass spectrometry (left) and kinetics of CFTRact-J027 concentration in plasma determined by liquid chromatography–mass spectrometry after bolus IP or oral administration of 10 mg/kg CFTRact-J027 at zero time (right, means ± SEM, 3 mice per group). (C) In vitro toxicity measured by Alamar Blue assay in FRT cells (means ± SEM; n = 3 cultures). (D) Body weight and lung wet/dry weight ratio in mice receiving 10 mg/kg CFTRact-J027 orally for 7 days (means ± SEM, 5 mice per group). (E) Chronic administration protocol (left) and efficacy of oral CFTRact-J027 after 7-day administration (means ± SEM, 5 mice per group). Student t test, *P < .05, **P < .01, ***P < .001. conc, concentration; Lop, loperamide.