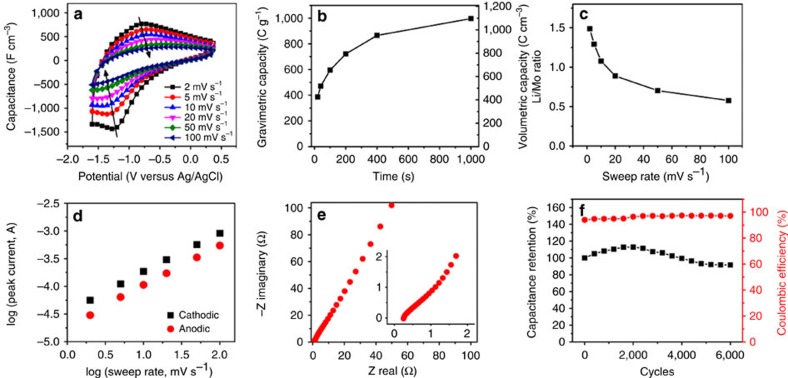
Figure 5. Electrochemical performance of 2D h-MoO3 in a Li-ion-containing organic electrolyte.
(a) CV curves at sweep rates ranging from 2 to 100 mV s−1 indicating the small peak shifts following the arrows. (b) Gravimetric and volumetric capacity as a function of charging/discharging time. (c) Li/Mo ratio at different sweep rates using x=QM/mF, where Q is the stored charge, M is the molecular weight, m is the mass and F is the Faraday constant. After 1,000 s, 2D h-MoO3 reaches 1.49 Li/Mo, approaching the theoretical value for bulk α-MoO3 (1.5). (d) Plot for calculating b values of the cathodic and anodic peaks (both=0.9). (e) Nyquist plots. (f) Cycling stability and Coulombic efficiency over 6,000 cycles.