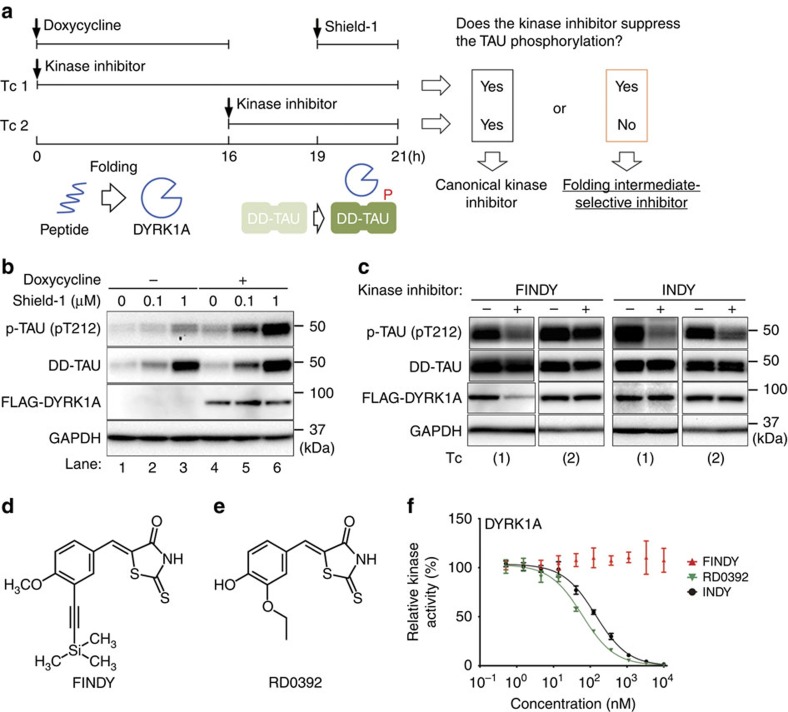
Figure 1. Cell-based assay to evaluate a transitional intermediate-selective inhibitor of DYRK1A.
(a) Schematic diagram of the SPHINKS assay. Doxycycline induces DYRK1A expression. Subsequently, Shield-1 stabilizes TAU fused with the destabilization domain of FKBP12 (DD-TAU). DYRK1A phosphorylates DD-TAU over the defined time period (19–21 h). Small molecules from our chemical library were added at the indicated points in Tc 1 and 2. Canonical kinase inhibitors suppress TAU phosphorylation in both Tc 1 and 2. Intermediate-selective inhibitors of DYRK1A should suppress TAU phosphorylation in Tc 1, but not in Tc 2. (b) Inducible expression of FLAG-DYRK1A and DD-TAU in HEK293 cells. Doxycycline induced FLAG-DYRK1A expression (lanes 4–6) and Shield-1 stabilized DD-TAU (lanes 2, 3, 5, 6). FLAG-DYRK1A predominantly phosphorylated Thr212 of TAU (p-TAU; lanes 5 and 6). p-TAU, TAU, FLAG and GAPDH were detected by western blot, using their corresponding antibodies. Representative data from the triplicate experiments are shown. (c) Identification of FINDY as the intermediate-selective inhibitor of DYRK1A. TAU phosphorylation was suppressed by FINDY (10 μM) in Tc 1, but not in Tc 2. In contrast, the canonical DYRK1A inhibitor INDY (10 μM) suppressed TAU phosphorylation in both Tc 1 and 2. Representative data from the triplicate experiments are shown. (d) Structure of FINDY. (e) Structure of RD0392, a canonical ATP-competitive inhibitor of DYRK1A. (f) FINDY did not inhibit the in vitro kinase activity of DYRK1A. Recombinant DYRK1A was incubated with the peptide substrate DYRKtide in the presence of FINDY, RD0392 or INDY. RD0392 and INDY inhibited the kinase activity with IC50 values of 60.2 and 139 nM, respectively. Representative dose-response curves with Hill slopes are shown. The results are presented as means±s.d. (n=4).