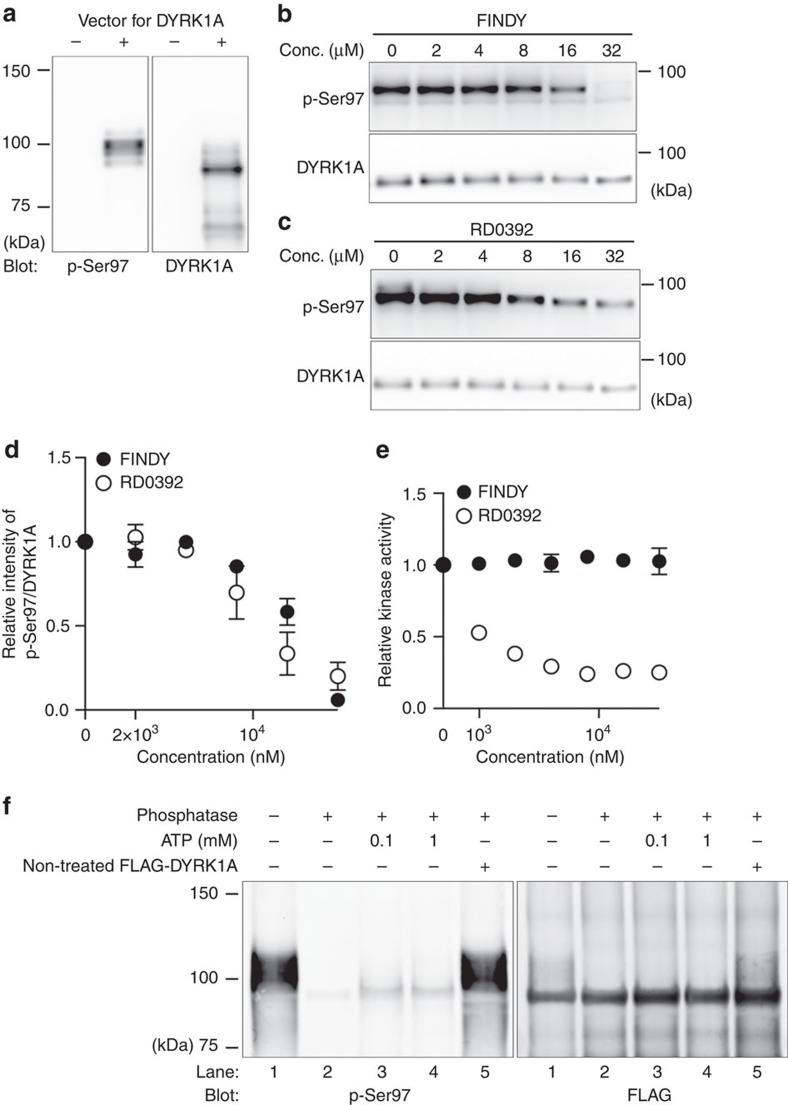
Figure 5. FINDY suppresses Ser97 autophosphorylation of DYRK1A in an in vitro transcription-translation system.
(a) Expression of DYRK1A in a cell-free E. coli-based coupled transcription-translation system. Representative data from the duplicate experiments are shown. (b,c) In vitro expression of DYRK1A in the presence of FINDY (b) or RD0392 (c). Representative data from the triplicate experiments are shown. (d) Relative activities of Ser97 autophosphorylation in the in vitro expression of DYRK1A in the presence of FINDY or RD0392. The band intensities in b,c were quantified. The graph shows the means±s.d. (n=3). (e) DYRK1A produced in the in vitro system was incubated with the peptide substrate DYRKtide in the presence of FINDY or RD0392 (up to 32 μM). The results are presented as means±s.d. (n=3). (f) DYRK1A produced in the in vitro system was purified with anti-FLAG antibody-conjugated beads, then reacted with lambda protein phosphatase for 2 h. After washout of phosphatase, the dephosphorylated DYRK1A was allowed to autophosphorylate in the presence of ATP and sodium orthovanadate (1 mM) for 2 h. To check the residual phosphatase activity, the non-treated FLAG-DYRK1A protein was added into the autophosphorylation reaction (lane 5). Representative data from the duplicate experiments are shown. Conc., concentration.