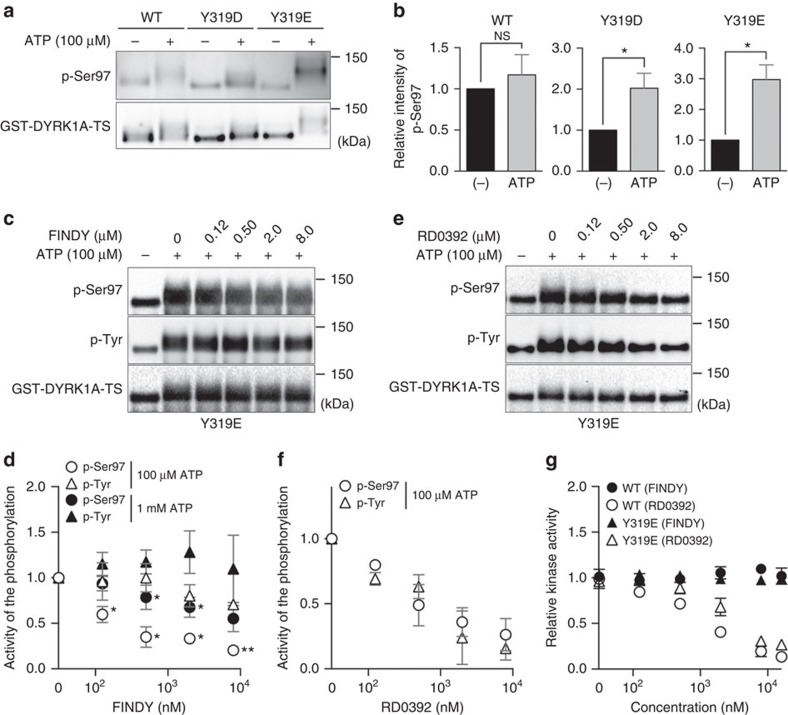
Figure 6. FINDY inhibits Ser97 autophosphorylation of recombinant DYRK1A protein.
(a) Recombinant GST-DYRK1A-TS protein (WT) and its pseudo-phosphorylation mutants (Y319D and Y319E), produced at 6 °C in E. coli, were reacted with ATP (100 μM), then analysed by SDS–PAGE followed by western blot analysis. Representative data from the quadruplicate experiments are shown. (b) The band intensities in a were quantified. The graph shows the means±s.d. (n=4). Statistical significance was calculated compared with the value without ATP. NS indicates not significant. *P<0.05. Statistical analysis was performed with the Mann–Whitney U-test. (c) The Y319E mutant was reacted with ATP (100 μM) in the presence of FINDY. Representative data from the triplicate experiments are shown. (d) Relative activities of Ser97 and tyrosine phosphorylation in the presence of ATP (100 μM and 1 mM) and FINDY, which were calculated from the ATP-dependent increase in the intensities of p-Ser97 and p-Tyr (see the Methods for details). The graph represents means±s.d. (n=3). Statistical significance was calculated compared with the value of the relative activity of p-Tyr at the same concentration of FINDY. *P<0.05 and **P<0.01. Data were analysed with the paired t-test. (e) RD0392 inhibited the phosphorylation of Ser97 and tyrosine residues. The Y319E mutant was reacted with ATP (100 μM) in the presence of RD0392. Representative data from the triplicate experiments are shown. (f) Relative activities of Ser97 and tyrosine phosphorylation in the presence of ATP (100 μM) and RD0392, which were calculated from the ATP-dependent increase in the intensities of p-Ser97 and p-Tyr (see the Methods for details). The graph represents means±s.d. (n=3). (g) The recombinant DYRK1A proteins were incubated with the peptide substrate DYRKtide in the presence of FINDY or RD0392 (up to 16 μM). The results are presented as means±s.d. (n=3).