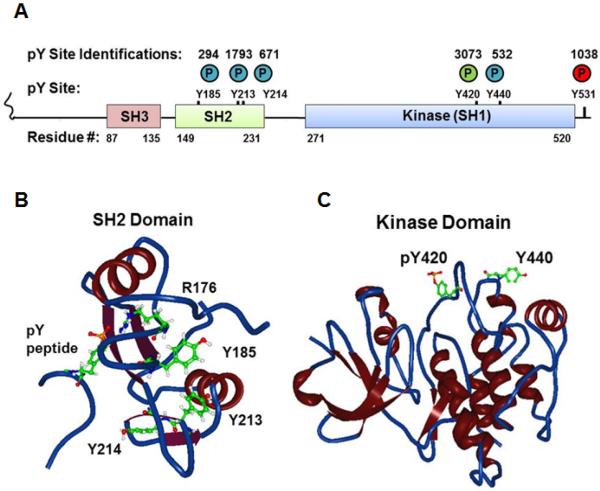
Fig. 1.
Localization of novel tyrosine phosphorylation sites (A) in a cartoon schema of the domain structure of human Fyn and in a backbone and ribbon 3D View rendering of structural data of (B) the Fyn SH2 domain with a phosphotyrosine peptide (PDB#1AOU) or (C) the Fyn kinase domain (PDB#2DQ7). Circled P denotes phosphate with colors corresponding to regulatory roles (green=positive; red=negative, blue=uncharacterized). Numbers above circles in (A) denote the number of independent identifications recorded in PhosphositePlus for each phosphorylation site. For the structural data atoms are portrayed of the phosphorylated tyrosine on the associated peptide; Arg176 and Tyr185, Tyr213, Tyr 214 on the SH2 domain; and the Fyn equivalents of phosphoTyr420 and Tyr440 on the kinase domain.