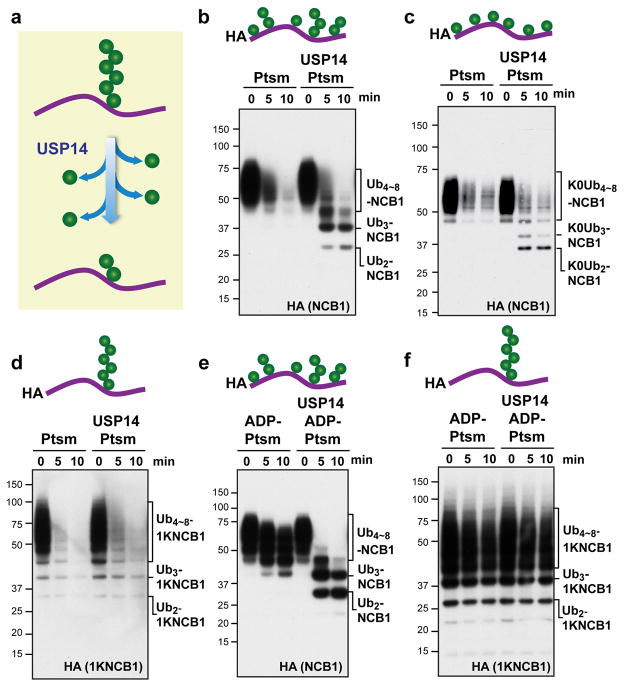
Figure 1. USP14 cleaves supernumerary ubiquitin chains from substrates.
a, Canonical model of USP14 activity. USP14 is thought to shorten ubiquitin chains progressively from their distal tip. b–f, In vitro degradation and deconjugation assays with polyubiquitinated N-terminal fragment of cyclin B1 (Ubn-NCB1; HA tagged). Cartoons show idealized representations of the expected distribution of ubiquitin moieties throughout. b, Human proteasome (Ptsm; 4 nM) was incubated with Ubn-NCB1 (~110 nM final) generated by UbcH10. Where indicated, USP14 (80 nM) was added. c, As in b, except using polyubiquitinated NCB1 generated with lysine-less Ub. d, As in b, except using polyubiquitinated conjugates on K64-only NCB1 (1KNCB1, unless otherwise noted), generated by UbcH10 and Ube2S. e, Ubn-NCB1 (~110 nM) was incubated with hexokinase-treated ADP-proteasome (ADP-Ptsm) in the presence or absence of USP14 (80 nM). f, As in e, except using polyubiquitinated K64-only NCB1. Samples were analysed by SDS–PAGE/immunoblotting for HA. For gel source data, see Supplementary Information.