Figure 4. Asymmetric hydrosilylation/hydroamination of enones.
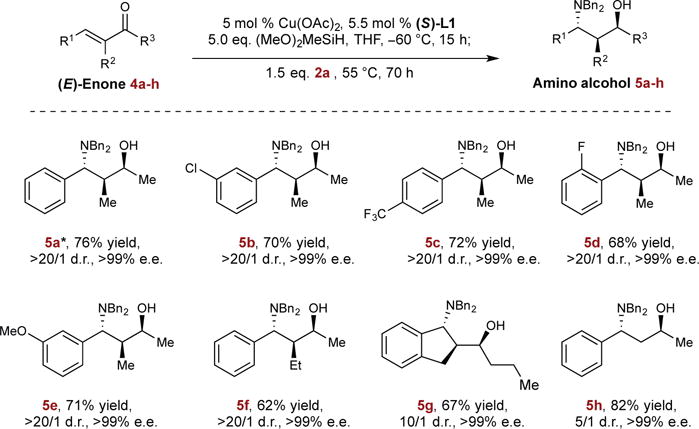
Top row, reaction studied; Bottom rows, substrate scope. Isolated yields are reported (average of two runs on 1.0 mmol scale). Diastereomeric ratios (d.r.) were determined by GC and NMR analysis. Enantiomeric excesses (e.e.) were determined by HPLC analysis. * The absolute and relative stereochemistry of 5a was determined to be (S,S,R) by single crystal X-ray diffraction. See Supplementary Information for details.
