Abstract
Background
Rhizospheric fungi play an essential role in the plant–soil ecosystem, affecting plant growth and health. In this study, we evaluated the fungal diversity in the rhizosphere soil of 2-yr-old healthy Panax notoginseng cultivated in Wenshan, China.
Methods
Culture-independent Illumina MiSeq and culture-dependent techniques, combining molecular and morphological characteristics, were used to analyze the rhizospheric fungal diversity. A diffusion test was used to challenge the phytopathogens of P. notoginseng.
Results
A total of 16,130 paired-end reads of the nuclear ribosomal internal transcribed spacer 2 were generated and clustered into 860 operational taxonomic units at 97% sequence similarity. All the operational taxonomic units were assigned to five phyla and 79 genera. Zygomycota (46.2%) and Ascomycota (37.8%) were the dominant taxa; Mortierella and unclassified Mortierellales accounted for a large proportion (44.9%) at genus level. The relative abundance of Fusarium and Phoma sequences was high, accounting for 12.9% and 5.5%, respectively. In total, 113 fungal isolates were isolated from rhizosphere soil. They were assigned to five classes, eight orders (except for an Incertae sedis), 26 genera, and 43 species based on morphological characteristics and phylogenetic analysis of the internal transcribed spacer. Fusarium was the most isolated genus with six species (24 isolates, 21.2%). The abundance of Phoma was also relatively high (8.0%). Thirteen isolates displayed antimicrobial activity against at least one test fungus.
Conclusion
Our results suggest that diverse fungi including potential pathogenic ones exist in the rhizosphere soil of 2-yr-old P. notoginseng and that antagonistic isolates may be useful for biological control of pathogens.
Keywords: fungal diversity, Illumina MiSeq, Panax notoginseng, rhizosphere soil
1. Introduction
Soil microbial communities display high structural, genetic, and functional diversity, and play extremely complex roles in many aspects of soil ecosystems, such as biotic interactions and nutrient cycling [1]. Microbial diversity in soil is an important factor determining soil health and is considered one of the main drivers in soil suppressiveness [2]. The rhizosphere is one of the most complex environments that are influenced by plant roots and active microhabitat where plant roots and microbes interact [3], [4]. Composition of the rhizosphere microbiota can negatively or positively influence plant traits such as stress tolerance, health, development, and productivity [5], [6]. Conversely, host plants are able to shape their own rhizosphere microbiome by adjusting environmental factors such as pH, soil nutrients, and root architecture [5], and recent studies have shown that plant genotype also has a great influence on the structure of rhizosphere microbial communities [7], [8], [9]. Fungi and fungus-like organisms form one of the most diverse groups of the Eukarya, and represent an essential functional component of soil microbial communities [10]. Under unfavorable conditions, some fungi can cause plant diseases and sometimes even total loss of the crop yields. In many instances, these diseases are caused by a complex of fungal species. At the same time, other fungal groups can antagonize phytopathogens, decompose plant residues, supply nutrients for plants, and stimulate plant growth [11].
The culture-dependent approach has been applied to analyze the fungal diversity for several decades [12]. In the past 20 yrs, many molecular methods, such as terminal-restriction fragment length polymorphism (T-RFLP), random amplified polymorphic DNA (RAPD), single-strand conformation polymorphism (SSCP), denaturing gradient gel electrophoresis (DGGE), and real-time quantitative PCR detecting system (Q-PCR), have widely been used to study the diversity and composition of soil microbial communities [13], [14]. With methodological advances, high-throughput sequencing techniques, such as 454 pyrosequencing and MiSeq by Illumina (San Diego, CA, USA), have been considered more powerful tools for estimating microbial communities [14], [15]. Compared to the 454 pyrosequencing, MiSeq sequencing has shorter time for generating good-quality paired-end reads and a much higher throughput at a low price [15], [16], [17]. The length of reads can reach 2 × 300 bp. Due to lower costs and greater throughput, Illumina MiSeq sequencing is increasingly being utilized to estimate the diversity and composition of microbial communities in various ecosystems.
Panax notoginseng (Burk.) F. H. Chen (Araliaceae), a well-known traditional Chinese medicinal plant mainly cultivated in the mountainous areas in Yunnan and Guangxi, is widely used for the treatment of cardiovascular diseases, inflammation, trauma, and internal and external bleeding due to injury, and has also been used as a tonic [18], [19]. Grown under shaded conditions with high humidity for at least 3 yrs, cultivation of P. notoginseng is easily affected by the soil environment, and P. notoginseng is also susceptible to a number of soil-borne diseases [20]. Among the diseases, the root-rot disease complex caused by a single pathogen or a combination of pathogens is most destructive because it results in yield reduction, or no harvest, and a lower content of active ingredients [21]. The reported pathogens include Alternaria panax, Alternaria tenuissima, Cylindrocarpon destructans, Cylindrocarpon didynum, Fusarium oxysporum, Fusarium solani, Phytophthora cactorum, Phoma herbarum, and Rhizoctonia solani [22]. Fungal pathogens, endophytes, and rhizosphere microbes of Panax ginseng and Panax quinquefolius have been reported [23], [24], [25], [26], [27]. Investigation on fungi associated with P. notoginseng has been focused on pathogens, and few reports were about the diversity of fungal community in the rhizosphere soil of P. notoginseng.
In this study, the diversity of the rhizospheric fungal community of 2-yr-old healthy P. notoginseng plants was characterized using a MiSeq sequencing platform (2 × 300 bp). The culturable fungal community was also analyzed by culture-dependent methods, and the antagonistic activities of the fungal isolates were also challenged by the pathogens, A. panax, F. oxysporum, F. solani, and P. herbarum, isolated from rotten roots of P. notoginseng. The further purposes include the following: (1) finding antagonistic fungi for the development of biocontrol agents for soil-borne diseases of P. notoginseng; (2) exploring the active substances from antagonistic fungal strains; and (3) investigating the fungal structure for in-depth studies on microbial ecological functions.
2. Materials and methods
2.1. Site and sampling
Soil samples were collected in a P. notoginseng plantation (N 23°35′, E 103°40′) in Wenshan, Yunnan, in early June 2013. Soil adhering around the roots was gently taken off, put into a sterile plastic bag, and transported to the laboratory within 24 h. Soil samples were preserved at −80°C and 4°C before use.
2.2. Soil DNA extraction and MiSeq sequencing
DNA was extracted from 0.5 g frozen soil sample using the PowerSoil DNA Isolation Kit (MO BIO Laboratories Inc., Carlsbad, CA, USA), according to the manufacturer's instructions. The nuclear ribosomal internal transcribed spacer 2 (ITS2) region was amplified according to the newly developed primers fITS9 and ITS4 [28]. Amplifications were carried out in a total volume of 50 μL using 10 ng of DNA, 5 μL 10 × polymerase chain reaction (PCR) buffer, 5 μL deoxy-ribonucleoside triphosphate (dNTP) (10mM each), 2.5 unit Plantium Taq, and 0.5 μL each of Bar-PCR primer (50μM) and Primer R (50μM). PCR cycling conditions were as follows: 5 min at 94°C; 25 cycles of 30 s at 94°C, 30 s at 58°C, 30 s at 72°C; and 7 min at 72°C. The PCR products were purified and run using a MiSeq Benchtop for 2 × 300 bp paired-end sequencing (Illumina). The quality check was performed using PRINSEQ-lite 0.19.5. All sequences were aligned using UCLUST v1.1.579 (http://www.drive5.com/uclust/downloads1_1_579.html), and complete linkage clustering was used to define operational taxonomic units (OTUs) with 97% identity as a cutoff. Rarefaction analysis was performed using MOTHUR v.1.22.2 [29]. Diversity in the sample was estimated using the Shannon–Weiner index and the species richness was expressed as the number of OTUs by the nonparametric indices abundance-based coverage estimate (ACE) and Chao1 [30]. In addition, taxonomy assignment was performed using the Ribosomal Database Project (RDP) classifier [31].
2.3. Isolation of fungi
Fungal isolation was carried out using the dilution method. Soil sample (10 g) was mixed with 90 mL sterile water in a conical flask, and the soil solution was diluted from 10−1 to 10−3 after shaking for 1 h in an oscillator. A volume of 0.1 mL soil solution (10−3) was coated evenly on potato dextrose agar with penicillin sodium salt (100 mg in 1 L medium) and kanamycin sulfate (50 mg in 1 L medium) to suppress bacterial growth. All plates were incubated at room temperature for 1 wk or until fungal growth was observed. Emergent hyphae were transferred and purified on sterile potato dextrose agar plates. Fungal isolates were grouped into morphotypes on the basis of their morphological differences.
2.4. Molecular identification and diversity analysis
Genomic DNA was extracted from pure cultures of a representative isolate of each fungal morphotype using the method of Guo et al [32]. The nuclear ribosomal internal transcribed spacer (ITS) regions including the 5.8S gene were subsequently amplified using the primer set ITS1 and ITS4 [33]. The amplification was performed in a total volume of 50 μL containing 10–50 ng of genomic DNA, 0.4μM of each primer, 0.2mM dNTP, 2.5 unit Easytaq DNA polymerase, and 5 μL 10× EasyTaq buffer (TransGen Biotech Co., Ltd, Beijing, China). Conditions for amplification were as follows: an initial denaturation step of 4 min at 94°C, followed by 35 cycles of 45 s at 94°C, 45 s at 55°C, 2 min at 72°C, and a final extension at 72°C for 10 min. Purified PCR products were directly sequenced with primer pairs as mentioned above in the ABI 3730-XL DNA sequencer (Applied Biosystems, Inc., Foster City, CA, USA). DNA sequences were analyzed with DNASTAR version 5.03 software (DNASTAR, Inc., Madison, WI, USA), and fungal identification was performed using the NCBI database (http://www.ncbi.nlm.nih.gov) and BLAST searches.
2.5. Determination of antagonistic fungi against pathogens of root-rot disease
Antimicrobial activities of isolated fungi were determined by the agar diffusion method. The root-rot pathogens A. panax, F. oxysporum, F. solani, and P. herbarum were used as the targets for antagonistic determination. Selected isolates were inoculated into 250 mL conical flasks, each with 50 mL of potato dextrose broth, and incubated at 28°C on a shaker at 170 rpm for 7 d. Then, 200 μL of culture suspension was added into a well (diameter: 5 mm) of the potato dextrose agar plate with pathogens. After incubation at 28°C for 4 d, the activity was expressed as the diameter of the growth inhibition zone (in mm). Each treatment was performed in triplicate.
3. Results
3.1. Fungal diversity and relative abundance in the rhizosphere soil of 2-yr-old P. notoginseng
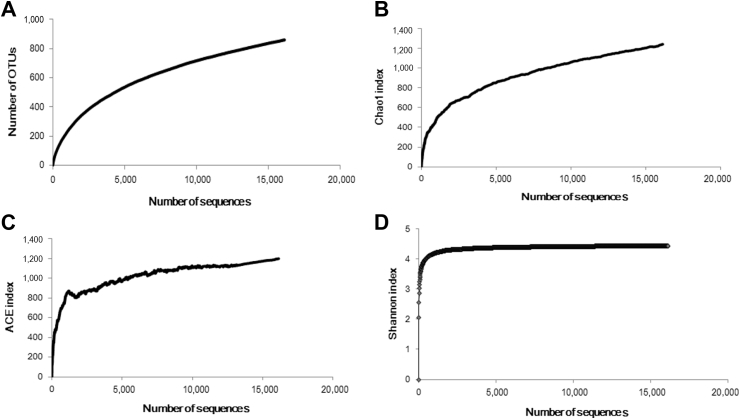
After quality filtering, a total of 16,130 paired-end reads were generated from 2-yr-old P. notoginseng rhizosphere soil sample using an Illumina MiSeq platform, and the sequencing coverage reached 0.98. The average length of these reads was 346 bp. All reads were clustered into 860 OTUs containing 564 non-singletons and 296 singletons at 97% sequence similarity. Rarefaction curves showed that the number of OTUs observed increased with the number of sequences sampled, and reached the near plateau region at 97% similarity level (Fig. 1A). The number of OTUs observed was still lower than the number of OTUs estimated with the nonparametric Chao1 (Fig. 1B) and ACE (Fig. 1C) indices (1,243 and 1,199, respectively) at 97% similarity. The Shannon diversity index (H′ = 4.44, Fig. 1D) showed that the fungi were highly diverse in 2-yr-old P. notoginseng rhizosphere soil.
Fig. 1.
Rarefaction curves showing the number of similarity-based OTUs at a cluster distance value of 0.03. Rarefaction analysis of (A) species richness, (B) Chao1 index, (C) ACE index, and (D) Shannon index. Four rarefaction curves reached the near plateau phase, representing good sampling depth.
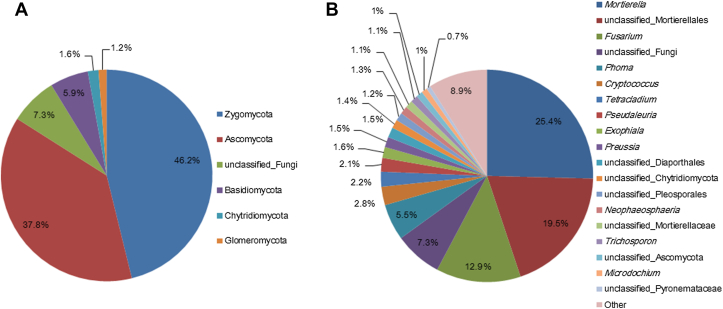
We assessed the distribution of fungal taxa at phylum and genus levels using the RDP classifier. They were assigned to five phyla and 79 genera. At phylum level, most fungal sequences were distributed in five phyla (Fig. 2A), Zygomycota (46.2%), Ascomycota (37.8%), Basidiomycota (5.9%), Chytridiomycota (1.6%), and Glomeromycota (1.2%), and others belonged to unclassified fungi. At genus level (Fig. 2B), Mortierella and unclassified Mortierellales accounted for a large proportion (44.9%). It is noteworthy that the relative abundance of Fusarium and Phoma sequences was high, accounting for 12.9% and 5.5%, respectively. Nearly all the Fusarium sequences were identified to be F. oxysporum, which was considered the major pathogen of root rot in P. notoginseng [22]. The taxa below 0.7% were grouped together and represented as “other” in Fig. 2B.
Fig. 2.
Proportional distribution of fungal taxa. Distribution of fungal (A) phyla and (B) genera present in rhizosphere soil of 2-year-old healthy Panax notoginseng, as revealed by MiSeq sequencing. “Other” in Fig. 2B represents all the taxa below 0.7%.
3.2. Identification and diversity of isolated fungi
A total of 113 fungal isolates were obtained from the rhizosphere soil of P. notoginseng; among them 43 morphotypes were recognizable based on their morphological characteristics. One isolate of each morphotype was used for phylogenetic analysis. ITS sequences of the 43 isolates were sequenced and blasted in GenBank, and the closest matches, the accession number in GenBank (Nos. KP230805–KP230847), are listed in Table 1. The phylogenetic analysis was performed with MEGA 5 program (http://www.megasoftware.net/); the resulting phylogenetic tree of all sequences obtained in this study, together with selected GenBank sequences of close relatives, is shown in Fig. 3.
Table 1.
The analysis of rhizospheric fungi associated with Panax notoginseng with the related species based on BLASTN search and their relative abundance
| Isolate | Closest species in GenBank (accession No.) | Sequence coverage (%) | Max. identity (%) | Taxon (accession no.) | Relative abundance (%) |
|---|---|---|---|---|---|
| Sordariomycetes | |||||
| PH30033 | Plectosphaerella sp. FJ430715 | 100 | 99 | Plectosphaerella sp. KP230834 | 3.54 |
| Plectosphaerella cucumerinaJQ796755 | 99 | 99 | |||
| PH30017 | Gibellulopsis nigrescensKC156644 | 99 | 100 | Gibellulopsis nigrescens KP230818 | 1.77 |
| PH30029 | Gliomastix masseeiAB540554 | 100 | 99 | Gliomastix masseei KP230830 | 2.66 |
| PH30019 | Trichoderma koningiopsisKF494183 | 100 | 99 | Trichoderma sp.1 KP230820 | 2.66 |
| Trichoderma gamsiiKJ680371 | 100 | 99 | |||
| PH30004 | Trichoderma harzianumFJ442660 | 100 | 100 | Trichoderma sp.2 KP230805 | 1.77 |
| Trichoderma aureovirideAF359400 | 100 | 100 | |||
| PH30008 | Paecilomyces marquandiiKM203617 | 100 | 99 | Paecilomyces marquandii KP230809 | 4.43 |
| PH30022 | Purpureocillium lilacinumFJ765024 | 100 | 99 | Purpureocillium lilacinum KP230823 | 0.88 |
| PH30018 | Cylindrocarpon sp. HQ637289 | 100 | 99 | Cylindrocarpon sp.1 KP230819 | 1.77 |
| PH30026 | Cylindrocarpon sp. AB725901 | 100 | 97 | Cylindrocarpon sp.2 KP230827 | 2.66 |
| PH30045 | Fusarium solaniHE974455 | 100 | 99 | Fusarium solani KP230846 | 5.31 |
| PH30025 | Fusarium chlamydosporumKF494035 | 100 | 100 | Fusarium chlamydosporum KP230826 | 3.54 |
| PH30010 | Fusarium oxysporumJX573316 | 100 | 100 | Fusarium oxysporum KP230811 | 5.31 |
| PH30012 | Fusarium tricinctumKJ598867 | 100 | 100 | Fusarium sp. KP230813 | 3.54 |
| Fusarium acuminatumKF887097 | 100 | 100 | |||
| PH30034 | Fusarium merismoidesAB586998 | 100 | 99 | Fusarium merismoides KP230835 | 0.88 |
| PH30043 | Fusarium flocciferumJN676123 | 100 | 100 | Fusarium flocciferum KP230844 | 2.66 |
| PH30011 | Volutella ciliataHQ703419 | 100 | 99 | Volutella ciliata KP230812 | 3.54 |
| PH30007 | Chaetomium megalocarpumKC109743 | 100 | 99 | Chaetomium sp. KP230808 | 2.66 |
| Chaetomium globosumJN209870 | 100 | 99 | |||
| PH30021 | Chaetomium globosumKF053680 | 100 | 99 | Chaetomium globosum KP230822 | 1.77 |
| PH30006 | Arthrinium arundinisKF144888 | 100 | 99 | Arthrinium sp. KP230807 | 0.88 |
| Arthrinium sacchariAB693901 | 100 | 99 | |||
| PH30020 | Pestalotiopsis cocculiEF055191 | 99 | 99 | Pestalotiopsis sp.1 KP230821 | 0.88 |
| Pestalotiopsis caudataEF055188 | 99 | 99 | |||
| PH30024 | Pestalotiopsis disseminataHQ607992 | 100 | 99 | Pestalotiopsis sp.2 KP230825 | 0.88 |
| Pestalotiopsis vismiaeEF055218 | 100 | 99 | |||
| Pestalotiopsis neglectaEF055222 | 100 | 99 | |||
| PH30031 | Pestalotiopsis lespedezaeGQ869901 | 100 | 100 | Pestalotiopsis lespedezae KP230832 | 1.77 |
| PH30044 | Pestalotiopsis neglectaEF055213 | 99 | 100 | Pestalotiopsis sp.3 KP230845 | 1.77 |
| Pestalotiopsis lespedezaeEF055206 | 99 | 100 | |||
| PH30046 | Sarocladium strictumGQ376096 | 100 | 100 | Sarocladium strictum KP230847 | 1.77 |
| Eurotiomycetes | |||||
| PH30035 | Penicillium chrysogenumAY373902 | 100 | 100 | Penicillium sp. KP230836 | 2.66 |
| Penicillium allii-sativiKJ775600 | 100 | 100 | |||
| PH30009 | Penicillium vancouverenseJN617675 | 100 | 99 | Penicillium vancouverense KP230810 | 1.77 |
| PH30005 | Aspergillus nidulansAY373888 | 100 | 100 | Aspergillus nidulans KP230806 | 2.66 |
| Chaetothyriomycetes | |||||
| PH30028 | Exophiala pisciphilaNR121269 | 100 | 99 | Exophiala pisciphila KP230829 | 2.66 |
| Dothideomycetes | |||||
| PH30027 | Microsphaeropsis arundinisJQ344344 | 100 | 99 | Microsphaeropsis arundinis KP230828 | 1.77 |
| PH30041 | Paraconiothyrium brasilienseKJ767099 | 92 | 98 | Paraconiothyrium sp. KP230842 | 0.88 |
| PH30037 | Paraconiothyrium cyclothyrioidesKC215138 | 100 | 100 | Paraconiothyrium cyclothyrioides KP230838 | 1.77 |
| PH30036 | Peyronellaea pinodellaKM030324 | 100 | 100 | Peyronellaea pinodella KP230837 | 0.88 |
| PH30030 | Phoma sp. JN207319 | 100 | 99 | Phoma sp.1 KP230831 | 1.77 |
| PH30038 | Phoma herbarumKJ767079 | 100 | 99 | Phoma herbarum KP230839 | 3.54 |
| PH30032 | Tetraplosphaeria sasicolaNR119404 | 99 | 97 | Tetraplosphaeria sp. KP230833 | 0.88 |
| PH30039 | Phaeosphaeriopsis sp. JQ936185 | 100 | 99 | Phaeosphaeriopsis sp. KP230840 | 1.77 |
| PH30040 | Phoma sp. JQ238617 | 94 | 95 | Phoma sp.2 KP230841 | 0.88 |
| PH30013 | Phoma medicaginisKF850377 | 99 | 98 | Phoma sp.3 KP230814 | 1.77 |
| PH30023 | Pyrenochaeta sp. HQ914829 | 100 | 99 | Pyrenochaeta sp. KP230824 | 0.88 |
| PH30016 | Alternaria tenuissimaKM921667 | 100 | 100 | Alternaria sp.2 KP230817 | 3.54 |
| Alternaria alternataKM371730 | 100 | 100 | |||
| PH30015 | Cladosporium cladosporioidesHQ832794 | 100 | 100 | Cladosporium cladosporioides KP230816 | 2.66 |
| PH30014 | Cladosporium cladosporioidesKJ589542 | 100 | 100 | Cladosporium sp. KP230815 | 1.77 |
| Cladosporium tenuissimumKJ152168 | 100 | 100 | |||
| Zygomycetes | |||||
| PH30042 | Mortierella alpinaFJ025167 | 100 | 100 | Mortierella alpina KP230843 | 6.19 |
| Shannon diversity index (H′) | 3.62 | ||||
| Species richness (S) | 43 | ||||
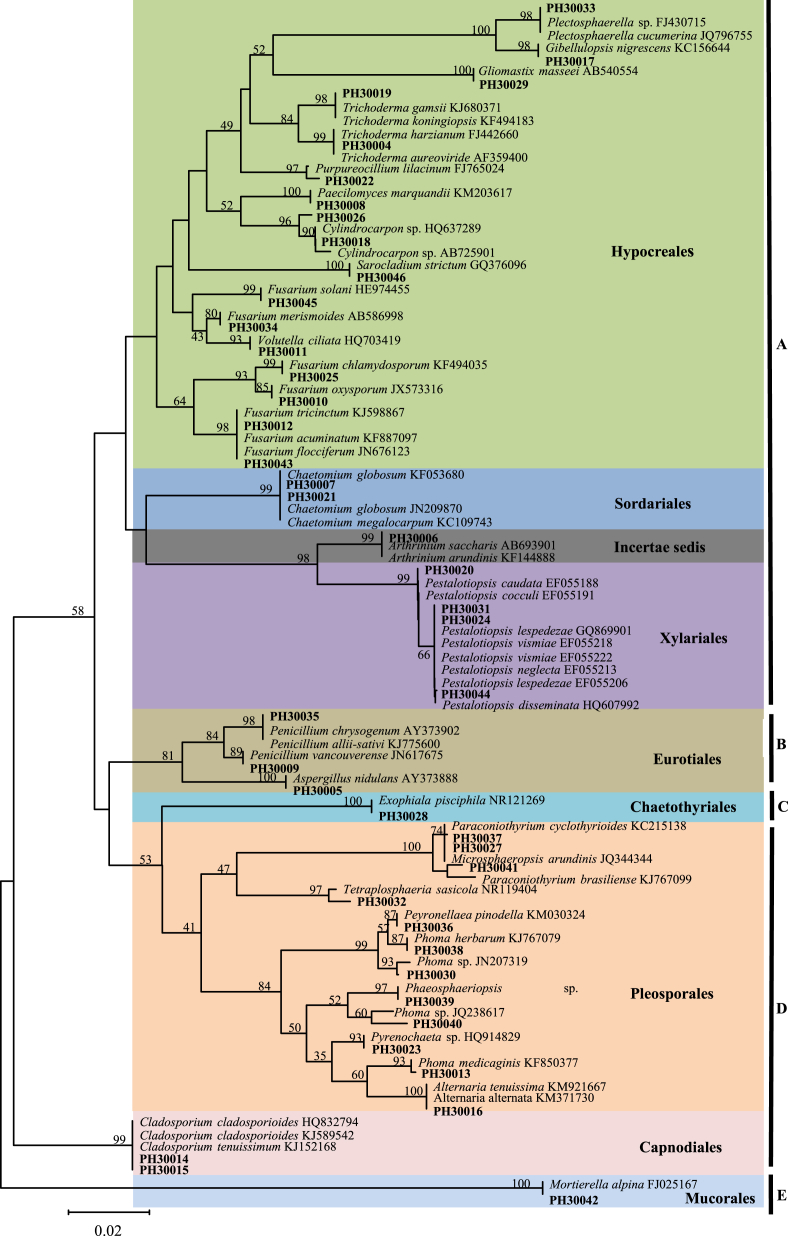
Fig. 3.
Neighbor-joining analysis of ITS sequences of 43 taxa from Panax notoginseng showing the relationships of culturable fungi with closest relatives. Bootstrap values are shown at the branches (1,000 replicates). The name of some groups is shown in bold letters: A, Sordariomycetes; B, Eurotiomycetes; C, Chaetothyriomycetes; D, Dothideomycetes; and E, Zygomycetes.
Based on the phylogenetic analysis of the 99 sequences (43 isolates and 56 sequences from GenBank), the isolates were distributed into two major groups. Forty-two isolates belonging to ascomycetes were grouped together in the first group, and the second group consisted of one zygomycetesisolate (PH30042). Five different fungal subgroups were identified, with four corresponding to Ascomycota − Sordariomycetes, Eurotiomycetes, Chaetothyriomycetes, and Dothideomycetes; and one subgroup belonging to Zygomycota, Zygomycetes. Sordariomycetes was the most dominant class (59.3%) comprising three orders (Hypocreales, Sordariales, and Xylariales) and a fungal strain of Incertae sedis (PH30006), and 23 species. Dothideomycetes (24.76%) included two orders, Pleosporales and Capnodiales, and 14 species.
Among the 43 identified fungal isolates, Mortierella alpina, F. oxysporum, and F. solani were the dominant species, which accounted for 6.19%, 5.31%, and 5.31% of the total isolates, respectively. Fusarium was the most isolated genus with 24 isolates (21.24%) and also the most diverse genus with six species, followed by Phoma and Pestalotiopsis, representing 7.96% and 5.3% of the total isolates (each with 4 species), respectively. Although Mortierella accounted for 6.19% of the total isolates, the genus contained only one species. Species richness (S = 43) and Shannon diversity index (H′ = 3.62) showed that the fungal species diversity was high in the rhizosphere soil of 2-yr-old P. notoginseng (Table 1).
3.3. Distribution of fungi with antimicrobial activity
Thirty-five of the 43 fungal isolates were challenged by four pathogenic fungi causing root rot of P. notoginseng, excepting eight potential pathogenic fungi, PH30010, PH30011, PH30018, PH30026, PH30033, PH30038, PH30043, and PH30045. Thirteen isolates (37.1%) displayed antimicrobial activity against at least one pathogenic fungus (Table 2). The antagonists were distributed in nine genera, mainly in Pestalotiopsis (3 species), Cladosporium (2 species), Trichoderma (2 species), and Chaetomium (2 species) (Table 2). Of the 13 isolates, eight were active against A. panax, 10 demonstrated activity against F. oxysporum, five displayed activity against F. solani, and only one showed weak activity against P. herbarum. Isolate PH30019 exhibited antimicrobial activity against all pathogen fungi tested. Four isolates (PH30007, PH30016, PH30019, and PH30035) exhibited moderate or strong antimicrobial activity against F. oxysporum and F. solani.
Table 2.
Antagonistic activities of fungal isolates against pathogenic fungi of Panax notoginseng
| solate No. | Diameter of the growth inhibition zone (mm) |
|||
|---|---|---|---|---|
| Alternaria panax | Fusarium oxysporum | Fusarium solani | Phoma herbarum | |
| PH30004 | 10 | 8 | ||
| PH30007 | 10 | 11 | ||
| PH30014 | 7 | 8 | ||
| PH30015 | 8 | 8 | ||
| PH30016 | 12 | 14 | ||
| PH30017 | 11 | |||
| PH30019 | 9 | 13 | 15 | 7 |
| PH30020 | 11 | |||
| PH30021 | 9 | 9 | ||
| PH30024 | 10 | |||
| PH30027 | 12 | |||
| PH30031 | 9 | |||
| PH30035 | 12 | 10 | 10 | |
4. Discussion
Fungal diversity analysis using high-throughput sequencing techniques is becoming more common [34]. Compared to 454 pyrosequencing, Illumina sequencing has more advantages under the same effect [15], [35]. Furthermore, the MiSeq platform can generate longer paired-end reads (2 × 300 bp). To our knowledge, a few studies have used MiSeq as a sequencing platform to study diversity and community composition of microbes [36], [37], [38]. Therefore, in this study, this new sequencing technique was employed to analyze the fungal diversity in the rhizosphere soil of P. notoginseng for the first time. A total of 16,978 raw reads were generated from the sequencer, and 16,130 sequences (95%) passed the quality filtering. A total of 860 OTUs were identified using a 97% cutoff during sequence clustering. The number of OTUs observed was lower than that of OTUs estimated with the nonparametric ACE and Chao1 indices at 97% similarity. Zygomycota was the dominant fungal phylum and represented 46.2% of all fungal DNA sequences, in which Mortierella accounted for a high relative abundance. Mortierella is ubiquitous in the bulk and rhizosphere soil from a range of temperate and tropical forests and agricultural habitats. A large number of Mortierella species exist in the rhizosphere soil of P. notoginseng, inferring that they play a potential role in keeping microecological balance as protective microbes to suppress soil-borne pathogens by competing for nutrition, or assisting the host plants with the uptake of phosphorus and nitrogen. Curlevski et al [39] suggested that Mortierella spp. might be an important component of phosphorus cycling.
DNA barcoding is increasingly applied to catalogue and classify biodiversity [40]. Generally, in culture-independent researches, fungal diversity is studied based on the analysis of either the small subunit or the ITS region of the nuclear ribosomal RNA gene. Owing to its higher rate of evolution than the small subunit fragment, the ITS region has been selected as the universal barcode for fungal identification [41]. Due to length constraints of high-throughput metabarcoding, early metabarcoding studies were restricted to the use of either ITS1 or ITS2 [42], [43]. However, because of the difference between the rates of evolution of ITS1 and ITS2, the composition of fungal communities characterized by ITS1 or ITS2 may be different. Bazzicalupo et al [40] suggested that ITS2 may be more variable and recovers higher operational taxonomic riches than ITS1, although both fragments performed equally well when evaluating community structures. Monard et al [34] proposed that ITS1 appeared to be a better choice for sequencing, and analysis of both ITS regions were complementary. In addition, selection of the primer is very important to reduce the quantity of the out-targeted sequences and chimeras. Ihrmark et al [28] evaluated the new primers—fITS7, gITS7, and fITS9—together with the ITS4 primer to amplify the ITS2 region by 454 sequencing. Primers fITS7 and fITS9 yielded a lower proportion of nonfungal sequences than gITS7. However, the fITS7 primer mismatched against most sequences of Mucorales. Mucoralean fungi are widely dispersed and occur in various habitats as saprobes in most ecosystems, such as soil, dung, dead plant material, and water [44]. Therefore, in this study, we analyzed the fungal diversity in the rhizosphere soil of P. notoginseng by amplifying the fungal ITS2 region using the fITS9f/ITS4 primer combination. The dataset obtained from the Illumina MiSeq platform was basically satisfactory and also supported the conclusion proposed by Ihrmark et al [28]
In pure-culture experiments, we isolated 113 fungal strains from the rhizosphere soil of 2-yr-old P. notoginseng. Based on the phylogenetic analysis and morphological characteristics, the isolates were assigned to five classes, eight orders (except for an I. sedis), 26 genera, and 43 species (Table 1; Fig. 3). Chen et al [27] reported that Penicillium, Trichoderma, and Fusarium were the dominant fungal populations in ginseng rhizosphere soil in northeastern China. The composition, quality, and diversity distribution of soil fungi showed great differences between ginseng and notoginseng, but the proportion of pathogens was high in both plant rhizosphere soils. As evident from the results of culture-independent experiments, the proportion of Fusarium and Phoma is high, suggesting that the plant is in danger of pathogen invasion, although it appears to be healthy at the sampling time (early in June). Root-rot diseases of P. notoginseng usually occur heavily in July and August, as environmental and weather conditions are optimal for the pathogens. The relatively high frequency of Fusarium and Phoma may give some interpretation. At the same time, antagonistic isolates were also detected and obtained during this study. The antagonists may be useful in inhibiting or suppressing the growth of potential root-rot pathogenic fungi in the soil ecological systems. In our previous work, we found a fungus Aspergillus versicolor (PH30001) from the rhizosphere soil of 1-yr-old healthy P. notoginseng, which produced antagonistic compounds against F. solani [45]. Further in-depth studies are needed to investigate the ecological effects of the antagonists. Cylindrocarpon species were the major pathogens causing root rot of P. ginseng and P. quinquefolius [46], [47]. Miao et al [22] reported that C. destructans and C. didynum caused root rot of P. notoginseng with weak pathogenicity but wide distribution. Two Cylindrocarpon isolates (PH30018 and PH30026) were also obtained during this study. However, Cylindrocarpon was not detected in high-throughput sequencing tests. For this case, it may be associated with primer design or various quality-control approaches or database selection. U'Ren et al [48] also confirmed that primer selection influenced pyrosequencing-based inferences of diversity and community composition.
Most environmental microorganisms are unculurable in commonly used media and culture conditions. Rhizosphere is a unique environment, where beneficial microbes and pathogens have an important influence on the growth and health of plants [49]. Root exudates also play an important role in plant–microorganism interactions and functioning of the rhizosphere [50]. Moreover, fertilizers, fungicides, and other agrochemicals applied can also affect the composition and structure of soil microbial communities that may be closely linked with ecosystem functions [51], [52], [53]. These findings suggest that more attention should be devoted to changes in the microbial communities. Improving and combining pure-culture and culture-independent techniques can contribute to a better understanding of rhizosphere ecology. This will be helpful in improving the management of notoginseng planting.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported financially by the National Natural Science Foundation of China (no. 41361075), the Key Foundation Program of Yunnan Province of China (No. 2013FA015), the Major Program of Educational Commission of Yunnan Province of China (No. ZD2013008), and the Basic Research Foundation of Yunnan University (2013S204).
References
- 1.Bouffaud M.L., Kyselková M., Gouesnard B., Grunamann G., Muller D., Moënne-Loccoz Y. Is diversification history of maize influencing selection of soil bacteria by roots? Mol Ecol. 2012;21:195–206. doi: 10.1111/j.1365-294X.2011.05359.x. [DOI] [PubMed] [Google Scholar]
- 2.Xu L., Ravnskov S., Larsen J., Nilsson R.H., Nicolaisen M. Soil fungal community structure along a soil health gradient in pea fields examined using deep amplicon sequencing. Soil Boil Biochem. 2012;46:26–32. [Google Scholar]
- 3.Mendes R., Garbeva P., Raaijmakers J.M. The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol Rev. 2013;37:634–663. doi: 10.1111/1574-6976.12028. [DOI] [PubMed] [Google Scholar]
- 4.Singh A.K., Singh M., Dubey S.K. Rhizospheric fungal community structure of a Bt brinjal and a near isogenic variety. J Appl Microbiol. 2014;117:750–765. doi: 10.1111/jam.12549. [DOI] [PubMed] [Google Scholar]
- 5.Kristin A., Miranda H. The root microbiota—a fingerprint in the soil? Plant Soil. 2013;370:671–686. [Google Scholar]
- 6.Lakshmanan V., Selvaraj G., Bais H.P. Functional soil microbiome: belowground solutions to an aboveground problem. Plant Physiol. 2014;166:689–700. doi: 10.1104/pp.114.245811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aira M., Gómez-Brandón M., Lazcano C., Bååth E., Domínguez J. Plant genotype strongly modifies the structure and growth of maize rhizosphere microbial communities. Soil Biol Biochem. 2010;42:2276–2281. [Google Scholar]
- 8.Berendsen R.L., Pieterse C.M.J., Bakker P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012;17:478–486. doi: 10.1016/j.tplants.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Peiffer J.A., Spor A., Koren O., Jin Z., Tringe S.G., Dang J.L., Buckler E.S., Ley R.E. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc Natl Acad Sci U S A. 2013;110:6548–6553. doi: 10.1073/pnas.1302837110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buée M., Reich M., Murat C., Morin E., Nilsson R.H., Uroz S., Martin F. 454 Pyrosequencing analyses of forest soils reveal an unexpectedly high fungal diversity. New Phytol. 2009;184:449–456. doi: 10.1111/j.1469-8137.2009.03003.x. [DOI] [PubMed] [Google Scholar]
- 11.Sugiyama A., Ueda Y., Takase H., Yazaki K. Pyrosequencing assessment of rhizosphere fungal communities from a soybean field. Can J Microbiol. 2014;60:687–690. doi: 10.1139/cjm-2014-0443. [DOI] [PubMed] [Google Scholar]
- 12.Gomes N.C., Fagbola O., Costa R., Rumjanek N.G., Buchner A., Mendona-Hagler L., Smalla K. Dynamics of fungal communities in bulk and maize rhizosphere soil in the tropics. Appl Environ Microbiol. 2003;69:3758–3766. doi: 10.1128/AEM.69.7.3758-3766.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rincon-Florez V.A., Carvalhais L.C., Schenk P.M. Culture-independent molecular tools for soil and rhizosphere microbiology. Diversity. 2013;5:581–612. [Google Scholar]
- 14.Aravindraja C., Viszwapriya D. Karutha Pandian S. Ultra deep 16S rRNA sequencing analysis of geographically similar but diverse unexplored marine samples reveal varied bacterial community composition. PLOS One. 2013;8:e76724. doi: 10.1371/journal.pone.0076724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt P.A., Bálint M., Greshake B., Bandow C., Römbk J., Schmitt I. Illumina metabarcoding of a soil fungal community. Soil Biol Biochem. 2013;65:128–132. [Google Scholar]
- 16.Arumugam R., Chan X.Y., Yin W.F., Choo S.W., Chan K.G. Metagenomic analysis of microbial diversity of tropical sea water of Georgetown Coast, Malaysia. Life Sci J. 2013;1:2392–2396. [Google Scholar]
- 17.Fadrosh D.W., Ma B., Gajer P., Sengamalay N., Ott S., Brotman R.M., Ravel J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome. 2014;2:6. doi: 10.1186/2049-2618-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun H.X., Ye Y.P., Pan H.J., Pan Y.J. Adjuvant effect of Panax notoginseng saponins on the immune responses to ovalbumin in mice. Vaccine. 2004;22:3882–3889. doi: 10.1016/j.vaccine.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Guo H.B., Cui X.M., An N., Cai G.P. Sanchi ginseng (Panax notoginseng (Burkill) F. H. Chen) in china: distribution, cultivation and variations. Genet Resour Crop Evol. 2010;57:453–460. [Google Scholar]
- 20.Ma L., Cao Y.H., Cheng M.H., Huang Y., Mo M.H., Wang Y., Yang J.Z., Yang F.X. Phylogenetic diversity of bacterial endophytes of Panax notoginseng with antagonistic characteristics towards pathogens of root-rot disease complex. Antonie van Leeuwenhoek. 2013;103:299–312. doi: 10.1007/s10482-012-9810-3. [DOI] [PubMed] [Google Scholar]
- 21.Sun Y., Ke J., Ma N., Chen Z., Wang C., Cui X. Effects of root rot on saponin content in Panax notoginseng. Zhong Yao Cai. 2004;27:79–80. [PubMed] [Google Scholar]
- 22.Miao Z.Q., Li S.D., Liu X.Z., Chen Y.J., Li Y.H., Wang Y., Xia Z.Y., Zhang K.Q. The causal microorganisms of Panax notoginseng root rot disease. Sci Agric Sin. 2006;39:1371–1378. [Google Scholar]
- 23.Park Y.H., Kim Y.C., Park S.U., Lim H.S., Kim J.B., Cho B.K., Bae H. Age-dependent distribution of fungal endophytes in Panax ginseng roots cultivated in Korea. J Ginseng Res. 2012;36:327–333. doi: 10.5142/jgr.2012.36.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eo J.K., Choi M.S., Eom A.H. Diversity of endophytic fungi isolated from Korean ginseng leaves. Mycobiology. 2014;42:147–151. doi: 10.5941/MYCO.2014.42.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xing X., Guo S., Fu J. Biodiversity and distribution of endophytic fungi associated with Panax quinquefolium L. cultivated in a forest reserve. Symbiosis. 2010;51:161–166. [Google Scholar]
- 26.Punja Z.K. Fungal pathogens of American ginseng (Panax quinquefolium) in British Columbia. Can J Plant Pathol. 1997;19:301–306. [Google Scholar]
- 27.Chen X., Sun X.D., Bi S.Y., Lu G.Z. Fungi diversity of ginseng rhizosphere soil in northeastern China. Agric Sci Technol Hunan. 2010;11:132–136. [Google Scholar]
- 28.Ihrmark K., Bödeker I.T., Cruz-Martinez K., Friberg H., Kubartova A., Schenck J., Strid Y., Stenlid J., Brandström-Durling M., Clemmensen K.E. New primers to amplify the fungal ITS2 region—evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol Ecol. 2012;8:2666–2677. doi: 10.1111/j.1574-6941.2012.01437.x. [DOI] [PubMed] [Google Scholar]
- 29.Schloss P.D., Westcott S.L., Ryabin T., Hall J.R., Hartmann M., Hollister E.B., Lesniewski R.A., Oakley B.B., Parks D.H., Robinson C.J. Introducing mothur: open source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chao A., Chazdon R.L., Colwell R.K., Shen T.J. A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol Lett. 2005;8:148–159. [Google Scholar]
- 31.Cole J.R., Wang Q., Cardenas E., Fish J., Chai B., Farris R.J., Kulam-Syed-Mohideen A.S., McGarrell D.M., Marsh T., Garrity G.M. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37:D141–D145. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo L.D., Hyde K.D., Liew E.C.Y. Identification of endophytic fungi from Livistona chinensis based on morphology and rDNA sequences. New Phytol. 2000;147:617–630. doi: 10.1046/j.1469-8137.2000.00716.x. [DOI] [PubMed] [Google Scholar]
- 33.White T.J., Bruns T., Lee J., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR protocols: a guide to methods and applications. Academic Press; San Diego, CA, USA: 1990. pp. 315–322. [Google Scholar]
- 34.Monard C., Gantner S., Stenlid J. Utilizing ITS1 and ITS2 to study environmental fungal diversity using pyrosequencing. FEMS Microbiol Ecol. 2013;84:165–175. doi: 10.1111/1574-6941.12046. [DOI] [PubMed] [Google Scholar]
- 35.Luo C., Tsementzi D., Kyrpides N., Read T., Konstantinidis K.T. Direct comparisons of Illumina vs. Roche 454 sequencing technologies on the same microbial community DNA sample. PLoS One. 2012;7:e30087. doi: 10.1371/journal.pone.0030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snelling T.J., Genç B., McKain N., Watson M., Waters S.M., Creevey C.J., Wallace R.J. Diversity and community composition of methanogenic archaea in the rumen of Scottish upland sheep assessed by different methods. PLoS One. 2014;9:e106491. doi: 10.1371/journal.pone.0106491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z., Yang Y., He T., Xie S. Change of microbial community structure and functional gene abundance in nonylphenol-degrading sediment. Appl Microbiol Biotechnol. 2015;99:3259–3268. doi: 10.1007/s00253-014-6222-5. [DOI] [PubMed] [Google Scholar]
- 38.Caporaso J.G., Lauber C.L., Walters W.A., Berg-Lyons D., Huntley J., Fierer N., Owens S.M., Betley J., Fraser L., Bauer M. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Curlevski N.J.A., Xu Z.H., Anderson I.C., Cairney J.W.G. Diversity of soil and rhizosphere fungi under Araucaria bidwillii (Bunya pine) at an Australian tropical montane rainforest site. Fungal Divers. 2010;40:12–22. [Google Scholar]
- 40.Bazzicalupo A.L., Bálint M., Schmitt I. Comparison of ITS1 and ITS2 rDNA in 454 sequencing of hyperdiverse fungal communities. Fungal Ecol. 2013;6:102–109. [Google Scholar]
- 41.Schoch C.L., Seifert K.A., Huhndorf S., Robert V., Spouge J.L., Levesque C.A., Chen W., Consortium F.B. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proc Natl Acad Sci U S A. 2012;109:6241–6246. doi: 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jumpponen A., Jones K.L. Massively parallel 454 sequencing indicates hyperdiverse fungal communities in temperate Quercus macrocarpa phyllosphere. New Phytol. 2009;184:438–448. doi: 10.1111/j.1469-8137.2009.02990.x. [DOI] [PubMed] [Google Scholar]
- 43.Cordier T., Robin C., Capdevielle X., Desprez-Loustau M.L., Vacher C. Spatial variability of phyllosphere fungal assemblages: genetic distance predominates over geographic distance in a European beech stand (Fagus sylvatica) Fungal Ecol. 2012;5:509–520. [Google Scholar]
- 44.De Azevedo Santiago A.L., Dos Santos P.J., Maia L.C. Mucorales from the semiarid of Pernambuco, Brazil. Braz J Microbiol. 2013;44:299–305. doi: 10.1590/S1517-83822013005000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu K., Zheng Y.K., Miao C.P., Xiong Z.J., Xu L.H., Guan H.L., Yang Y.B., Zhao L.X. The antifungal metabolites obtained from the rhizospheric Aspergillus sp. YIM PH30001 against pathogenic fungi of Panax notoginseng. Nat Prod Res. 2014;28:2334–2337. doi: 10.1080/14786419.2014.935941. [DOI] [PubMed] [Google Scholar]
- 46.Yan X.R., Fu J.F. A summary of researches on Cylindrocarpon and Panax root rust-rot. J Shenyang Agric Univ. 2002;33:71–75. [Google Scholar]
- 47.Bai R.L., Liu X.M., Liu W.C. The fungal species causing root rot of ginseng in Jilin Province. Acta Phytopathol Sin. 1999;29:285. [Google Scholar]
- 48.U’Ren J.M., Riddle J.M., Monacell J.T., Carbone I., Miadlikowska J., Aronold A.E. Tissue storage and primer selection influence pyrosequencing-based inferences of diversity and community composition of endolichenic and endophytic fungi. Mol Ecol Resour. 2014;14:1032–1048. doi: 10.1111/1755-0998.12252. [DOI] [PubMed] [Google Scholar]
- 49.Lynch J. Wiley; London, UK: 1990. The rhizosphere. [Google Scholar]
- 50.Haichar F.Z., Santaella C., Heullin T., Achouak W. Root exudates mediated interactions belowground. Soil Biol Biochem. 2014;77:69–80. [Google Scholar]
- 51.Yu C., Hu X.M., Deng W., Li Y., Xiong C., Ye C.H., Han G.M., Li X. Changes in soil microbial community structure and functional diversity in the rhizosphere surrounding mulberry subjected to long-term fertilization. Appl Soil Ecol. 2014;86:30–40. [Google Scholar]
- 52.Schmitt H., Van Beelen P., Tolls J., Van Leeuwen C.L. Pollution-induced community tolerance of soil microbial communities caused by the antibiotic sulfachloropyridazine. Environ Sci Technol. 2004;38:1148–1153. doi: 10.1021/es034685p. [DOI] [PubMed] [Google Scholar]
- 53.Thiele-Bruhn S., Beck I.C. Effects of sulfonamide and tetracycline antibiotics on soil microbial activity and microbial biomass. Chemosphere. 2005;59:457–465. doi: 10.1016/j.chemosphere.2005.01.023. [DOI] [PubMed] [Google Scholar]