Abstract
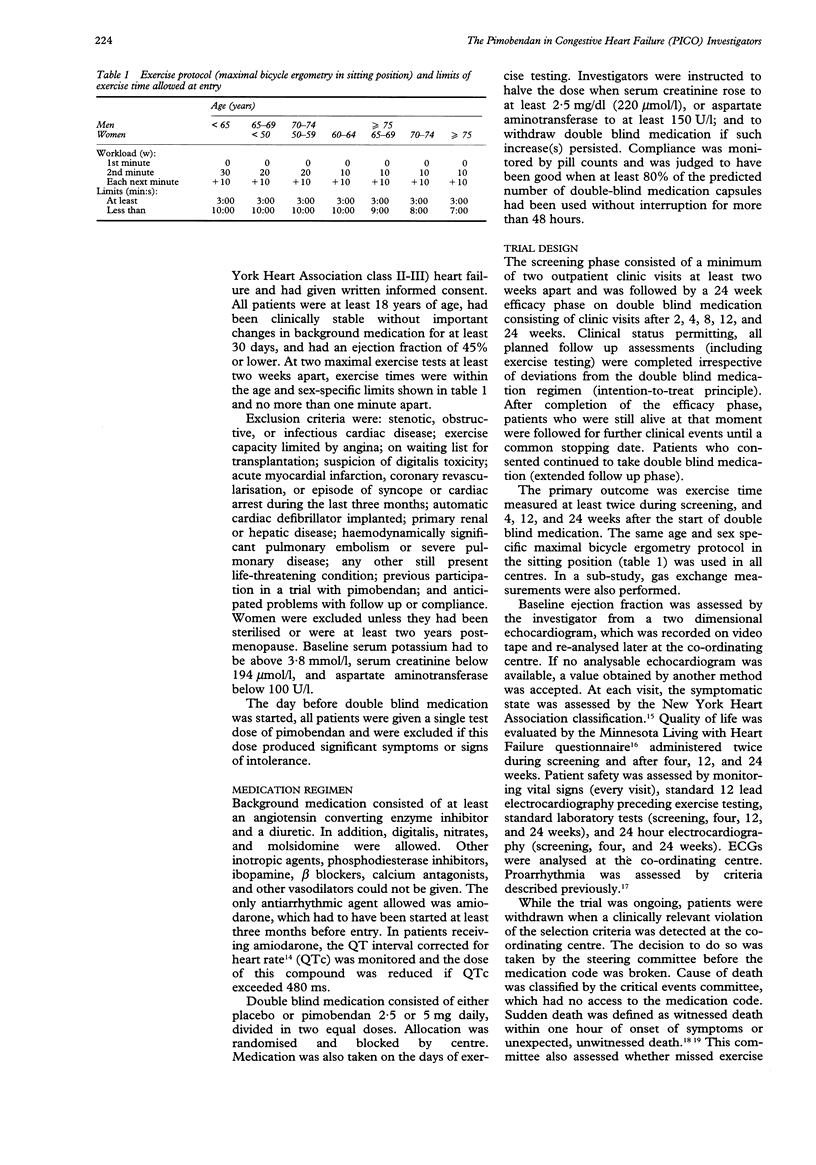
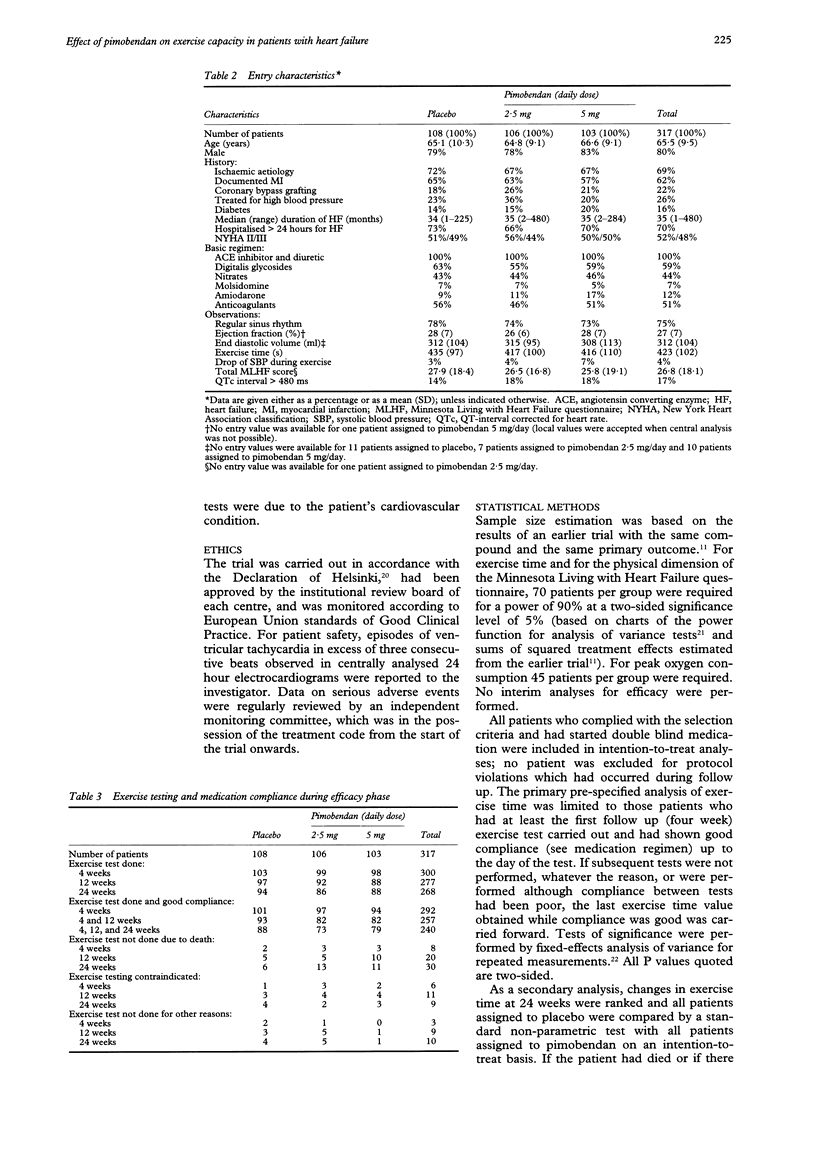
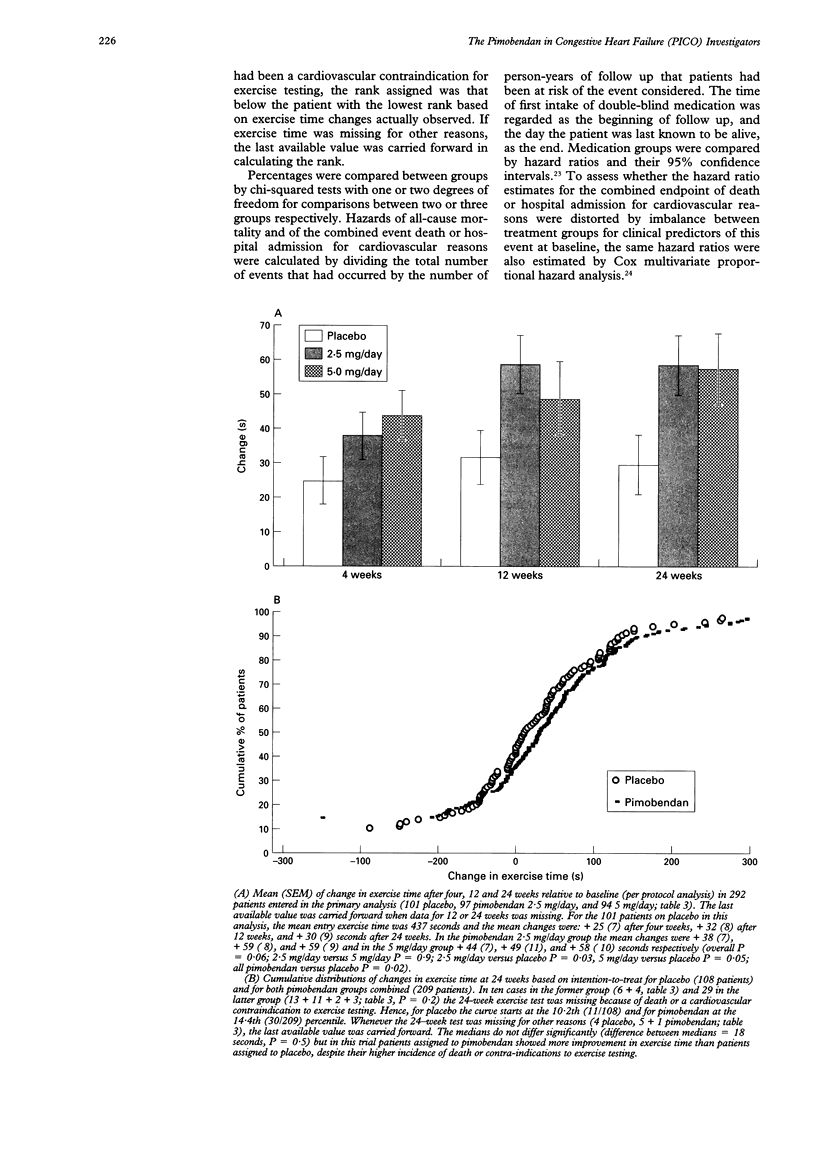
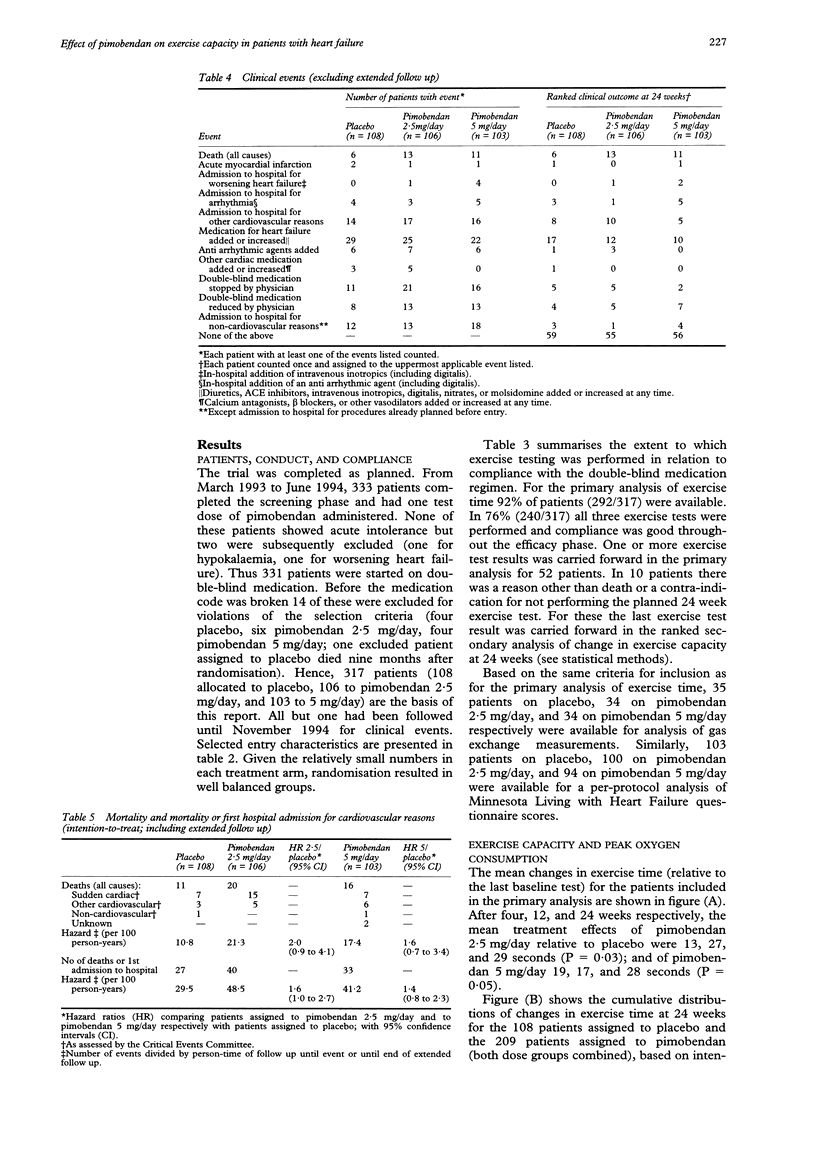
PRIMARY OBJECTIVE: To determine the effects of pimobendan 2.5 and 5 mg daily on exercise capacity in patients with chronic heart failure. DESIGN: A randomised, double blind, placebo controlled trial of the addition of pimobendan to conventional treatment with a minimum follow up of 24 weeks. SETTING: Outpatient cardiology clinics in six European countries. PATIENTS: 317 patients with stable symptomatic heart failure, objectively impaired exercise capacity, and an ejection fraction of 45% or lower who were treated with at least an angiotensin converting enzyme inhibitor and a diuretic and who tolerated a test dose of pimobendan. RESULTS: Compared with placebo, both pimobendan 2.5 and 5 mg daily improved exercise duration (bicycle ergometry) by 6% (P = 0.03 and 0.05) after 24 weeks of treatment. At that time 63% of patients allocated to pimobendan and 59% of those allocated to placebo were alive and able to exercise to at least the same level as at entry (P = 0.5). No significant effects on oxygen consumption (assessed in a subgroup of patients) and on quality of life (assessed by questionnaire) were observed. Pimobendan was well tolerated. Proarrhythmic effects (24-hour electrocardiography) were not observed. In both pimobendan groups combined the hazard of death was 1.8 (95% confidence interval 0.9 to 3.5) times higher than in the placebo group. CONCLUSIONS: Pimobendan improves exercise capacity in patients with chronic heart failure who are also on conventional treatment. The balance between benefit and risk of treatment with this compound remains to be established however.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colucci W. S., Wright R. F., Braunwald E. New positive inotropic agents in the treatment of congestive heart failure. Mechanisms of action and recent clinical developments. 2. N Engl J Med. 1986 Feb 6;314(6):349–358. doi: 10.1056/NEJM198602063140605. [DOI] [PubMed] [Google Scholar]
- Feldman A. M. Classification of positive inotropic agents. J Am Coll Cardiol. 1993 Oct;22(4):1223–1227. doi: 10.1016/0735-1097(93)90441-3. [DOI] [PubMed] [Google Scholar]
- Hagemeijer F. Calcium sensitization with pimobendan: pharmacology, haemodynamic improvement, and sudden death in patients with chronic congestive heart failure. Eur Heart J. 1993 Apr;14(4):551–566. doi: 10.1093/eurheartj/14.4.551. [DOI] [PubMed] [Google Scholar]
- Hasenfuss G., Holubarsch C., Heiss H. W., Just H. Influence of UDCG-115 on hemodynamics and myocardial energetics in patients with idiopathic dilated cardiomyopathy. Am Heart J. 1989 Sep;118(3):512–519. doi: 10.1016/0002-8703(89)90266-4. [DOI] [PubMed] [Google Scholar]
- Katz S. D., Kubo S. H., Jessup M., Brozena S., Troha J. M., Wahl J., Cohn J. N., Sonnenblick E. H., LeJemtel T. H. A multicenter, randomized, double-blind, placebo-controlled trial of pimobendan, a new cardiotonic and vasodilator agent, in patients with severe congestive heart failure. Am Heart J. 1992 Jan;123(1):95–103. doi: 10.1016/0002-8703(92)90752-h. [DOI] [PubMed] [Google Scholar]
- Kubo S. H., Gollub S., Bourge R., Rahko P., Cobb F., Jessup M., Brozena S., Brodsky M., Kirlin P., Shanes J. Beneficial effects of pimobendan on exercise tolerance and quality of life in patients with heart failure. Results of a multicenter trial. The Pimobendan Multicenter Research Group. Circulation. 1992 Mar;85(3):942–949. doi: 10.1161/01.cir.85.3.942. [DOI] [PubMed] [Google Scholar]
- Kulka P. J., Tryba M. Inotropic support of the critically ill patient. A review of the agents. Drugs. 1993 May;45(5):654–667. doi: 10.2165/00003495-199345050-00003. [DOI] [PubMed] [Google Scholar]
- Lubsen J. Exercise testing as outcome in congestive heart failure trials. Design considerations when interpreting results. Drugs. 1994;47 (Suppl 4):25–30. doi: 10.2165/00003495-199400474-00005. [DOI] [PubMed] [Google Scholar]
- Morganroth J., Borland M., Chao G. Application of a frequency definition of ventricular proarrhythmia. Am J Cardiol. 1987 Jan 1;59(1):97–99. doi: 10.1016/s0002-9149(87)80078-4. [DOI] [PubMed] [Google Scholar]
- Narang R., Swedberg K., Cleland J. G. What is the ideal study design for evaluation of treatment for heart failure? Insights from trials assessing the effect of ACE inhibitors on exercise capacity. Eur Heart J. 1996 Jan;17(1):120–134. doi: 10.1093/oxfordjournals.eurheartj.a014670. [DOI] [PubMed] [Google Scholar]
- PEARSON E. S., HARTLEY H. O. Charts of the power function for analysis of variance tests, derived from the non-central F-distribution. Biometrika. 1951 Jun;38(1-2):112–130. [PubMed] [Google Scholar]
- Packer M., Carver J. R., Rodeheffer R. J., Ivanhoe R. J., DiBianco R., Zeldis S. M., Hendrix G. H., Bommer W. J., Elkayam U., Kukin M. L. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med. 1991 Nov 21;325(21):1468–1475. doi: 10.1056/NEJM199111213252103. [DOI] [PubMed] [Google Scholar]
- Remme W. J., Krayenbühl H. P., Baumann G., Frick M. H., Haehl M., Nehmiz G., Baiker W. Long-term efficacy and safety of pimobendan in moderate heart failure. A double-blind parallel 6-month comparison with enalapril. The Pimobendan-Enalapril Study Group. Eur Heart J. 1994 Jul;15(7):947–956. doi: 10.1093/oxfordjournals.eurheartj.a060615. [DOI] [PubMed] [Google Scholar]
- Sasayama S., Asanoi H., Kihara Y., Yokawa S., Terada Y., Yoshida S., Ejiri M., Horikoshi I. Clinical effects of long-term administration of pimobendan in patients with moderate congestive heart failure. Heart Vessels. 1994;9(3):113–120. doi: 10.1007/BF01745236. [DOI] [PubMed] [Google Scholar]
- Uretsky B. F., Jessup M., Konstam M. A., Dec G. W., Leier C. V., Benotti J., Murali S., Herrmann H. C., Sandberg J. A. Multicenter trial of oral enoximone in patients with moderate to moderately severe congestive heart failure. Lack of benefit compared with placebo. Enoximone Multicenter Trial Group. Circulation. 1990 Sep;82(3):774–780. doi: 10.1161/01.cir.82.3.774. [DOI] [PubMed] [Google Scholar]


