Abstract
Background
Whether prehospital point‐of‐care (POC) troponin further accelerates the time to diagnosis in patients with chest pain (CP) is unknown. We conducted a randomized trial of POC‐Troponin testing in the ambulance.
Methods and Results
Patients with chest pain presenting by ambulance were randomized to usual care (UC) or POC‐Troponin; ST‐elevation myocardial infarction patients or those with noncardiovascular symptoms were excluded. Pre‐hospital high‐sensitivity troponin was analyzed on a POC device and available to the paramedic and emergency department (ED) staff. The final diagnosis was centrally adjudicated. The primary endpoint was time from first medical contact to discharge from ED or admission to hospital. We randomized 601 patients in 19 months; 296 to UC and 305 to POC‐Troponin. After ambulance arrival, the first troponin was available in 38 minutes in POC‐Troponin and 139 minutes in UC. In POC‐Troponin, the troponin was >0.01 ng/mL in 17.4% and >0.03 ng/mL in 9.8%. Patients spent a median of 9.0 hours from first medical contact to final disposition, and 165 (27.4%) were admitted to the hospital. The primary endpoint was shorter in patients randomized to POC‐Troponin (median 8.8 hours [6.2–10.8] compared to UC (median 9.1 hours [6.7–11.2]; P=0.05). There was no difference in the secondary endpoint of repeat ED visits, hospitalizations, or death in the next 30 days.
Conclusions
In this broad population of patients with CP, ambulance POC‐Troponin accelerated the time to final disposition. Enhanced and more cost‐effective early ED discharge of the majority of patients with CP calling 911 is an unrealized opportunity.
Clinical Trial Registration
URL: https://www.ClinicalTrials.gov/. Unique identifier: NCT01634425.
Keywords: acute coronary syndromes, ambulance, chest pain, clinical trial, troponin
Subject Categories: Clinical Studies, Acute Coronary Syndromes, Biomarkers
Acute cardiovascular disease (CVD) constitutes a major resource intensive public health challenge. Many patients with chest discomfort present by ambulance and are taken to the emergency department (ED) for further diagnosis and treatment, leading to frequent investigations, longer times in the ED, and associated increased health care costs.1, 2, 3 The majority of these patients are ultimately discovered to have a noncardiac cause for their chest discomfort, leading to speculation that these patients could be more effectively and rapidly managed.
Troponin testing in the ED is the standard of care for patients with chest discomfort to “rule out” an acute cardiac event.4, 5 Recent advances in the sensitivity of troponin has led to a focus on earlier, more precise detection of events.6 Recently, a 2‐hour ED algorithm was found to be efficient and safe for ruling out acute coronary syndromes.7 Hence, moving proximally to an earlier time point for diagnostic testing deserves exploration. Prehospital testing of troponin in cohort studies have shown a sensitivity ranging from 31% to 87% and a negative predictive value of up to 100% for the diagnosis of an acute coronary syndrome.8, 9, 10 Because many patients with chest discomfort activate emergency medical services and present by ambulance, point‐of‐care (POC) testing beginning earlier in the care pathway provides the potential for more rapid triage of patients with suspected acute coronary syndrome.
In a previous trial, Providing Rapid Out of Hospital Acute Cardiovascular Treatment 3 (PROACT‐3), we found that prehospital testing of B‐type natriuretic peptide (BNP) or POC‐Troponin did not shorten time from first medical contact to final disposition in patients with a broad range of cardiovascular symptoms.11 However, some insights into the specific subset of patients with chest pain (CP) were gained. First, we enrolled a broad cohort of patients and tested 2 biomarkers (BNP and troponin), which may have diminished our ability to see a difference if one existed because of heterogeneity of symptoms, patients, and final diagnosis. Second, we demonstrated that in our urban environment, patients spend between 8 and 9 hours in an ambulance and the ED if they presented with CP. Finally, we identified that greater integration of information from the ambulance (including blood test results) to the multidisciplinary ED clinical team is required to ensure this information is dealt with appropriately.
Accordingly, the PROACT‐4 trial was designed to test whether POC‐Troponin would reduce the time from first medical contact to final ED patient disposition in patients activating the emergency medical services (EMS) system with acute chest discomfort.
Methods
Study Design
PROACT‐4 was a prospective, open‐label, blinded‐endpoint (PROBE) randomized, clinical trial. Patients were randomized in the prehospital setting 1:1 into POC‐Troponin or usual care (UC) groups.
Participants and Setting
Eligible patients were adults over age 30 years of age that activated prehospital EMS for symptoms of acute chest discomfort for which acute CVD was deemed to be the most probable diagnosis by EMS personnel. Patients were excluded if they had documented ST elevation on the initial 12‐lead electrocardiogram (ECG) or with a previous diagnosis that was compatible with a noncardiovascular cause (eg, chest trauma, severe asthma), syncope or central nervous system symptoms, cardiac arrest, ventricular tachycardia, or atrial fibrillation with a heart rate >110 beats per minute. Patients provided verbal assent to paramedics on scene followed by written consent, as approved by the health research ethic board at the University of Alberta (Edmonton, Alberta, Canada).
The trial utilized prehospital Web‐based randomization with equal allocation in 25 ambulances within Edmonton, Alberta, Canada. Edmonton has a single integrated system of care (catchment area ≈1.1 million people) involving all hospitals and the prehospital system. Ambulances are staffed by 2 EMS personnel and are triaged by a central system. Within the region there are 5 hospitals (2 of which are tertiary care and are percutaneous coronary intervention [PCI]‐capable) and one free‐standing ED. Enrollment took place 24 hours a day, 7 days a week from July 2013 to February 2015, during which time the region had 31 ambulances and 373 paramedics responding to 4089 calls for CP.
Intervention
After obtaining informed consent in eligible patients, patients were randomly assigned to POC‐Troponin or UC. UC includes a standard assessment and plan per EMS protocols, including a history, physical, 12‐lead ECG, and starting at least 1 intravenous line. No POC blood tests were available in the ambulances for the duration of the study other than within the trial. In the POC‐Troponin arm, troponin was drawn while on scene (prehospital) and analyzed on a POC device in the ambulance. POC‐Troponin results were known to the EMS personnel once available and relayed to the treating ED team on arrival to the ED. The prehospital triage of patients to different hospitals was not altered by the protocol and in‐hospital assessment, and management was managed by existing standard of care. Treating physicians were encouraged to incorporate POC‐Troponin results into their diagnostic and treatment plan, and the use of a CP protocol was strongly encouraged. ED personnel were educated on the protocol, local ED physicians and cardiologist opinion leaders provided education to ED physicians, patients were provided wrist bands identifying their participation in the trial, and POC‐troponin results were additionally provided in a highly visible manner on the patient chart.
Troponin Assay
The POC Triage device (Alere, San Diego, CA) provided a troponin result within ≈15 minutes on a digital and paper‐based readout. This was selected because it was established in our previous trial,11 was small enough for deployment in the ambulance, and both the assays and device are Health Canada approved. Plasma troponin I concentrations were measured by the Alere Cardio2 panel (analytical sensitivity is 0.01 ng/mL with a 99th percentile [male and female] of 0.02 ng/mL).12 The positive test value for POC‐Troponin was prespecified at >0.03 ng/mL. The in‐hospital troponin I assay was run on the Beckman AccuTnI assay (analytical sensitivity is 0.01 ng/mL with a 99th percentile [male and female] of 0.04 ng/mL). In‐hospital troponin in our region does not clinically report (or make available) troponin values ≤0.1 ng/mL; thus, we only report troponin values >0.1 ng/mL.
Outcomes
The primary endpoint was time from first medical contact to final disposition in the ED. First medical contact was defined as arrival of the paramedics on scene at the patient location. Final patient disposition was defined as the time when a plan for patient discharge from the ED or admission to hospital was documented. Secondary endpoints included 30‐day events (all‐cause mortality, all‐cause hospitalization or rehospitalization, or all‐cause re‐ED visits) were captured by primary electronic and paper chart review. If there were multiple events for an individual patient, only the first event was counted. There was 100% capture of primary and secondary endpoints.
Adjudication of Diagnosis
An adjudication committee (made up of members of the executive and steering committee with experience and expertise in clinical practice) adjudicated the diagnosis blinded to the randomized assignment using definitions based on national and international guidelines. The adjudication committee chair reviewed the case and assigned a diagnosis in the event of a disagreement. In order to replicate the clinical scenario, only information from the ED was available to adjudicators, including ED physician notes, consult notes, admission orders, ECG, and hospital‐based labs; the POC‐Troponin results from the ambulance were not available to the adjudicators.13 The final diagnosis of patients was further summarized into 4 categories: angina; acute coronary syndrome; acute heart failure; and “other.”
Blinding
Patients, EMS personnel and physicians treating the patient were aware of the allocated arm; however, outcome assessors, the adjudication committee, and data analysts were blinded to the allocation.
Sample Size
In estimating the sample size for PROACT‐4, we relied on data from PROACT‐3.11 In PROACT‐3, a median time from ambulance arrival to final disposition was 8.8 hours (6.2–10.6 hours; with log transformation for non‐normal data, geometric mean=2.129, SD=0.472).14 A 2‐sided alpha of 0.05, 90% power, and SDs of equal size over the logarithmic‐transformed time data, and a 25% relative reduction (120 minutes) in the POC‐Troponin arm compared to UC, required 283 patients per arm. No dropouts were anticipated (given the intervention occurs on scene in the ambulance immediately after randomization). We presumed a 10% device or sample failure rate, missing data, and protocol deviation, and therefore we increased the sample size to 300 patients per arm, or 600 patients total.
Statistical Analysis
Continuous variables were reported as median and 25th and 75th percentiles, whereas categorical variables were reported as counts or percentages, as noted. Differences among patient characteristics, timing intervals, and biomarkers between the UC and biomarker study arms were tested by the Wilcoxon rank‐sum test for continuous variables and by the chi‐square test or Fisher's exact test for categorical variables.
The primary endpoint was time from first medical contact to final disposition (minutes). For analysis of the difference in this time interval between UC and POC‐Troponin study arms, the distribution of this metric was assessed first. Given violation of normal distribution assumption for both original and transformed time interval, the nonparametric Mann–Whitney test was used. To account for the unbalance of the 2 arms, modified GRACE score (age, heart rate, systolic blood pressure, creatinine, cardiac arrest at admission, elevated cardiac enzymes, and Killip class) was used to adjust the primary endpoint.15, 16 A quantile regression model was used to perform the adjustment, and an adjusted P value is reported. For the secondary endpoints, the clinical event rates in each study arm were compared using the chi‐square test or Fisher's exact test when cell number was less than 5. Both intention‐to‐treat (ITT; ie, random assignment to POC‐Troponin vs UC) and per protocol analyses (ie, patient received biomarker test) were performed for primary and secondary endpoints comparison.
Sensitivity, specificity, positive predictive value, and negative predictive value were examined for POC‐Troponin relative to the adjudicated diagnosis of acute coronary syndrome (ACS), in patients randomized to the POC‐Troponin study arm. The positive test value for POC‐Troponin was prespecified at >0.03 ng/mL.
All analyses were performed using SAS software (version 9.4; SAS Institute Inc., Cary, NC), and all statistical tests were 2‐sided and had a 5% level of significance; there was no adjustment for multiple comparisons.
The Canadian VIGOUR Center (www.vigour.ualberta.ca) handled overall trial management, data collection and management, randomization, quality assurance, data queries, and data analysis. The health research ethic board at the University of Alberta approved this study. The trial is registered at ClinicalTrials.gov (NCT01634425). The authors had full access to, and take full responsibility for, the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Between July 8, 2013 and February 13, 2015, 601 patients were enrolled and randomized into PROACT‐4 (Figure). Clinical status was available on all patients for 30 days. After a 911 call, an ambulance arrived on scene a median of 7 minutes (25th–75th percentiles, 5–10 minutes) later. Paramedics were on scene longer in the POC‐Troponin arm (median of 31 minutes [25th–75th percentiles, 26–38 minutes]) compared to the UC arm (median of 27 minutes [25th–75th percentiles, 23–34 minutes]; P<0.001). After first medical contact, the first troponin was available a median of 38 minutes (25th–75th percentiles, 28–55 minutes) in patients randomized to POC‐Troponin, and hospital‐based troponin was available 138 minutes (25th–75th percentiles, 101–218 minutes; P<0.001) in patients in the UC arm.
Figure 1.
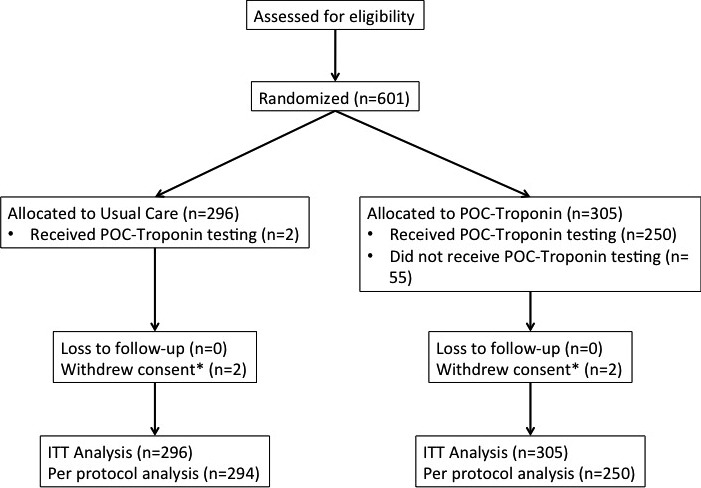
Patient accountability. ITT indicates intention to treat; POC, point‐of‐care.
Baseline patient demographics are shown in Table 1. The 2 groups were well balanced with a few exceptions. The POC‐Troponin arm had a lower percentage of patients with previous PCI and atrial fibrillation, a lower systolic blood pressure, and fewer patients >75 years of age. A total of 72.5% of patients were discharged home: This was similar between groups.
Table 1.
Selected Baseline Characteristics
| Usual Care | POC‐Troponin | P Value | |
|---|---|---|---|
| n | 296 | 305 | |
| Age, y | 68 (53, 79) | 64 (53, 76) | 0.138 |
| >75 y, n (%) | 98 (33.1) | 80 (26.2) | 0.065 |
| Female, n (%) | 136 (45.9) | 125 (41.0) | 0.220 |
| Vital signs in ambulance | |||
| Estimated weight, kg | 80 (70, 100) | 80 (70, 98) | 0.688 |
| Heart rate, beats per minute | 80 (72, 94) | 82 (70, 98) | 0.466 |
| Systolic blood pressure, mm Hg | 153 (137, 172) | 147 (131, 169) | 0.054 |
| Respiratory rate, breaths per minute | 18 (16, 20) | 18 (16, 20) | 0.589 |
| Past medical history, n (%) | |||
| Myocardial infarction | 82 (27.7) | 96 (31.5) | 0.311 |
| Previous percutaneous intervention | 48 (16.2) | 31 (10.2) | 0.028 |
| Previous coronary artery bypass graft | 23 (7.8) | 29 (9.5) | 0.449 |
| Angina | 40 (13.5) | 41 (13.4) | 0.980 |
| Heart failure | 29 (9.8) | 21 (6.9) | 0.196 |
| Atrial fibrillation | 29 (9.8) | 45 (14.8) | 0.065 |
| Diabetes | 72 (24.3) | 80 (26.2) | 0.591 |
| Hypertension | 181 (61.1) | 177 (58.0) | 0.437 |
| Hypercholesterolemia | 81 (27.4) | 77 (25.2) | 0.555 |
| Family history of CAD | 35 (11.8) | 29 (9.5) | 0.357 |
| Stoke or transient ischemic attack | 35 (11.8) | 31 (10.2) | 0.515 |
| COPD | 35 (11.8) | 39 (12.8) | 0.720 |
| POC‐troponin I, ng/mL, n (%) | |||
| ≤0.01 | — | 196 (64.3) | |
| >0.01 | — | 53 (17.4) | |
| ≤0.03 | — | 219 (71.8) | |
| >0.03 | — | 30 (9.8) | |
| Not done/missing | 56 (18.4) | ||
Values are median (25th–75th percentiles), unless otherwise stated. COPD indicates chronic obstructive pulmonary disease; POC, point‐of‐care.
The final adjudicated diagnosis was angina in 24 patients (4%), ACS in 112 patients (18.6%), acute heart failure (AHF) in 16 patients (3%), and “other” in 449 patients (73.5%; Table 2). The “other” group included 289 patients with CP not otherwise classified.
Table 2.
Adjudicated Diagnosis in Overall Trial Population
| Adjudicated Diagnosis | Sub‐Category of Adjudicated Diagnosis | N | Final Diagnosis | n |
|---|---|---|---|---|
| Angina | 24 | Angina | 24 | |
| Acute coronary syndromes | Unstable angina | 30 | Acute Coronary Syndromes | 112 |
| NSTEMI | 72 | |||
| STEMI | 10 | |||
| Acute heart failure | 16 | Acute Heart Failure | 16 | |
| Other cardiovascular | Myocarditis/Pericarditis | 2 | Other | 449 |
| Pulmonary embolism | 3 | |||
| Aortic dissection | 0 | |||
| Hypertrophic cardiomyopathy | 0 | |||
| Symptomatic aortic stenosis | 3 | |||
| Significant arrhythmia | 19 | |||
| Chest pain not otherwise classified | 289 | |||
| Pulmonary disease | COPD | 9 | ||
| Asthma | 1 | |||
| Acute Respiratory Infection | 15 | |||
| Pneumothorax | 0 | |||
| Gastrointestinal | GERD/PUD | 28 | ||
| Cholecystitis | 4 | |||
| Pancreatitis | 2 | |||
| Colitis | 1 | |||
| Musculoskeletal | Costochondritis | 0 | ||
| Musculoskeletal chest pain | 28 | |||
| Other | 45 |
COPD indicates chronic obstructive pulmonary disease; GERD, gastroesophogeal reflux disease; NSTEMI, non‐ST‐elevation myocardial infarction; PUD, peptic ulcer disease.
Troponin Results
A POC‐Troponin result was available in 252 (83%) of the 305 patients in the POC‐Troponin arm. Patients with or without a POC‐Troponin result were similar (Table 3).
Table 3.
Selected Baseline Characteristics of All Patients and Those Without POC‐Troponin Testing
| All | POC‐Troponin Tested | No POC‐Troponin Tested | |
|---|---|---|---|
| n | 601 | 252 | 55 |
| Age, y | 66 (53, 78) | 63 (53, 77) | 66 (54, 76) |
| >75 y, n (%) | 178 (30) | 67 (26.6) | 14 (25.5) |
| Female, n (%) | 261 (43.4) | 107 (42.5) | 19 (34.5) |
| Vital signs in ambulance | |||
| Estimated weight, kg | 80 (70, 100) | 80 (70, 99) | 82 (70, 97) |
| Heart rate, beats per minute | 81 (70, 96) | 81 (69, 96) | 84 (74, 100) |
| Systolic blood pressure, mm Hg | 151 (137, 170) | 148 (132, 169) | 147 (131, 170) |
| Respiratory rate, breaths per minute | 18 (16, 20) | 18 (16, 20) | 18 (16, 22) |
| Past medical history, n (%) | |||
| Diabetes | 152 (25.3) | 69 (27.4) | 12 (21.8) |
| Hypertension | 358 (59.6) | 149 (59.1) | 30 (54.5) |
| Hypercholesterolemia | 158 (26.3) | 64 (25.4) | 14 (25.5) |
| Previous angina | 81 (13.5) | 34 (13.5) | 7 (12.7) |
| Previous myocardial infarction | 178 (29.6) | 79 (31.3) | 18 (32.7) |
| Previous percutaneous intervention | 79 (13.1) | 25 (9.9) | 7 (12.7) |
| Previous coronary artery bypass graft | 52 (8.7) | 26 (10.3) | 4 (7.3) |
| Previous heart failure | 50 (8.3) | 17 (6.7) | 4 (7.3) |
| Previous COPD | 74 (12.3) | 33 (13.1) | 6 (10.9) |
| Family history of coronary artery disease | 64 (10.6) | 23 (9.1) | 7 (12.7) |
| Previous stoke | 66 (11) | 28 (11.1) | 3 (5.5) |
| Previous atrial fibrillation | 74 (12.3) | 35 (13.9) | 10 (18.2) |
Values are n (%) or median (25th–75th percentiles), unless otherwise stated. COPD indicates chronic obstructive pulmonary disease; POC, point‐of‐care.
Prehospital POC‐Troponin was >0.03 ng/mL in 9.8% and >0.1 ng/mL in 17.4% (Table 1). Of those patients with a POC‐Troponin >0.03 ng/mL, 9 patients were discharged home and 21 were admitted to the hospital. Of those patients with a POC‐Troponin >0.01 ng/mL, 23 patients were discharged home and 30 were admitted to the hospital. Using the prespecified threshold of a POC‐Troponin >0.03 ng/mL, no patient in the angina group, 22 (73.3%) in the ACS group, 2 (6.7%) in the AHF group, and 6 (20%) in the “other” group were above this threshold. In‐hospital troponin was >0.1 ng/mL in 11.8% (28 patients in UC and 43 patients in POC‐Troponin). Using the prespecified threshold of an initial in‐hospital troponin >0.1 ng/mL, no patients in the angina group, 55 (49%) in the ACS group, 3 (19%) in the AHF group, and 13 patients (3%) in the “other” group were above this threshold.
Primary and Secondary Endpoints
The primary endpoint (median time from first medical contact to final patient disposition) was 8.8 hours (6.2–10.8) in the POC‐Troponin group and 9.1 hours (6.7–11.2) in the UC group (P=0.05; P adjusted=0.059; Table 4). In patients who were discharged directly from the ED, the POC‐Troponin group had a shorter time from first medical contact to final disposition than those in the UC arm (P=0.035), however, there was no difference in those patients who were admitted to the hospital (P=0.621).
Table 4.
Primary and Secondary Endpoints
| Usual Care | POC‐Troponin | P Value | P Adjusteda | |
|---|---|---|---|---|
| N | 296 | 305 | ||
| Intention‐to‐treat analysis | ||||
| Primary endpoint | ||||
| First medical contact to final disposition, hours | 9.1 (6.7, 11.2) | 8.8 (6.2, 10.8) | 0.050 | 0.059 |
| Discharged from ED | 9.3 (7.4, 11.0) | 8.9 (6.7, 10.6) | 0.035 | 0.034 |
| Admitted to hospital | 8.7 (5.4, 12.0) | 8.2 (4.9, 12.3) | 0.621 | 0.535 |
| Secondary endpoints within 30 days, n (%) | ||||
| All‐cause death | 4 (1.4) | 4 (1.3) | 0.966 | |
| ED visit | 34 (11.6) | 43 (14.2) | 0.338 | |
| Rehospitalization | 18 (6.1) | 21 (6.9) | 0.690 | |
| ED visit or rehospitalization | 47 (16.0) | 59 (19.5) | 0.265 | |
| Per protocol analysis | 294 | 252 | ||
| First medical contact to final disposition, hours | 9.1 (6.7, 11.2) | 8.9 (6.2, 10.8) | 0.069 | 0.074 |
| Discharged from ED | 9.3 (7.4, 11.00) | 8.9 (6.7, 10.2) | 0.021 | 0.017 |
| Admitted to hospital | 8.7 (5.4, 12.0) | 8.6 (5.3, 12.6) | 0.959 | 0.908 |
| Secondary endpoints within 30 days, n (%) | ||||
| All‐cause death | 4 (1.4) | 3 (1.2) | 0.861 | |
| ED visit | 34 (11.6) | 34 (13.6) | 0.493 | |
| Rehospitalization | 18 (6.2) | 17 (6.8) | 0.764 | |
| ED visit or rehospitalization | 47 (16.1) | 46 (18.4) | 0.478 | |
Values are median (25th–75th percentiles), unless otherwise stated. P values are nonparametric Mann–Whitney test. BP indicates blood pressure; ED, emergency department; POC, point‐of‐care.
Adjusted for modified GRACE score (age, heart rate, systolic BP, creatinine, cardiac arrest at admission, elevated cardiac enzymes, Killip class)
In the per protocol analysis, the primary endpoint of first medical contact to final disposition trended to be shorter in the POC‐Troponin group, although this difference did not meet statistical significance (P=0.069; P adjusted=0.074). In patients who were discharged directly from the ED, the POC‐Troponin group had a shorter time from first medical contact to final disposition than those in the UC arm (P=0.021) and remained significant after adjustment (P=0.017); however, there was no difference between treatment groups for those patients who were admitted to hospital (P=0.959).
There were no differences in secondary outcomes of all‐cause death, repeat ED visits, or rehospitalization in the ITT or per‐protocol analysis.
Sensitivity, Specificity, and Positive and Negative Predictive Value
Using the threshold for POC‐Troponin of >0.03 ng/mL and examining the adjudicated diagnosis of ACS, compared to the other groups, with analysis limited to those patients with an available biomarker result, the sensitivity was 44%, specificity 96%, positive predictive value 73.3%, and negative predictive value 87.2%.
Discussion
CP, presenting by ambulance, forms a heterogeneous group of patients with cardiac and noncardiac disease requiring efficient ruling in or out of disease. In this randomized, controlled trial of POC‐Troponin testing in the ambulance, we identified a shorter time from first medical contact to final disposition in the ED. Given the broad range of patients’ final diagnoses, the rapidity of results, and the randomized design of the study, several conclusions can be drawn. First, POC‐Troponin testing in the ambulance is both feasible and could increase the efficiency of an ED using current‐generation troponin assays. Second, 10% of patients had troponin >0.03 ng/mL on their POC‐Troponin assay which could lead to more‐effective triage and treatment. Finally, and as with many biomarker studies in the acute setting, the majority of our patients were low risk, despite the presentation with CP and by ambulance, initial assessment by trained paramedics. This reinforces the need for health care systems to have efficient and cost‐effective strategies for this population.
Our study included a significant proportion (72.5%) who were discharged home directly from the ED. This group of patients in both the ITT and per protocol analysis, with and without adjustment for risk, had a greater association with reduced time in the POC‐Troponin group compared to UC. Patients discharged from the ED for later risk stratification or follow‐up care form the bulk of visits to the ED and thus an opportunity to start the diagnostic process even earlier. Ambulance systems are ideally suited to do this because they are staffed by trained health care personnel and have access (in most jurisdictions) to simple (eg, ECG) tools that could be integrated into a risk score to effectively triage and streamline these individuals to the appropriate level of care.
Similar to our previous study and to other biomarker testing studies in this population, there was no difference in clinical outcomes by either strategy in PROACT‐4.17, 18 PROACT‐4 was underpowered to detect a difference in the secondary clinical endpoints. The 30‐day mortality rate of 1.3% in the overall cohort and 4.5% in the ACS group is similar to previous studies of ED‐based algorithms.7, 17
The choice of troponin assay for this study was intended to balance the sensitivity with the specificity of the assay on a POC platform. The goal of this trial was to reduce the total time in a patient population who are predominantly low risk and are discharged home. A more sensitive assay (such as those recently endorsed by the European Society of Cardiology)19 may provide an efficient ruleout or rule‐in of an ACS, but have yet to be tested in an adequately powered randomized, controlled trial in broad populations, but rather in a prospective cohort design and in lower‐risk patients.20 Additionally, they have not been tested in the ambulance population of patients with CP and thus may provide different results if tested earlier in the health care environment. In our trial, 18% of patients did not have a POC‐Troponin result, which was because of device or kit failure, or difficulty getting intravenous access. Future work should consider the ambulance as a continuum of the health care environment, and use of rapid, validated, and portable algorithms and devices is worthy of study.
We chose to use the same endpoint we had used in PROACT‐3 because it reflects the total amount of time “exposed” to the health care system. It additionally provides a starting point for a randomized comparison unbiased by POC testing, thus enhancing its generalizability and applicability outside of the research environment. By applying an equal starting point, our endpoint (first medical contact to final disposition) crosses health care geographic boundaries (eg, EMS, patient scene, and ED) and could be measured across 5 different hospitals varying in size, volume, and expertise and thus is generalizable to other health care systems. Although the time difference in our primary endpoint fell short of the proposed 60 minutes, it still translates into a 23‐minute reduction in time by use of POC‐Troponin overall and 27 minutes (primary analysis) or 26 minutes (per‐protocol analysis) in those patients discharged from the ED. Moreover, during the 19‐month duration of the PROACT‐4 trial, this translates into roughly 1500 hours difference by use of a POC‐Troponin, if all patients with CP in the region were to be considered and thus has significant health care and cost implications.
Strengths and Limitations
Our study has both limitations and strengths that deserve consideration. First, trained paramedics identified patients in the ambulance with CP, such that the results would be generalizable to systems where this level of prehospital expertise exists. Paramedics in our region undergo extensive training, routine educational and certification, and are familiar with delivering advanced therapy (eg, fibrinolysis therapy for ST‐elevation myocardial infarction [STEMI]) in the ambulance. Hence, PROACT‐4 results should be placed in this context. In a jurisdiction with less‐advanced EMS systems, or greater distances or durations of EMS transport, there may be an even greater magnitude of the effect than we observed. Second, though we integrated our study into the ambulance with extensive training and continual quality assurance of devices, 18% did not have a POC‐Troponin result available. For this reason, we also preformed a per‐protocol analysis and the results were similar overall to the ITT analysis. We did not formally ascertain the degree with which ED physicians integrated the POC‐Troponin results into practice; however, this was strongly encouraged through education by local ED opinion leaders.
Conclusions
In this broad population of patients with CP, prehospital POC‐Troponin testing shortened the time from first medical contact to final disposition in the ED, and the magnitude of this effect was greatest in patients discharged from the ED. There was no difference in clinical outcomes. Future randomized trials testing the utility of POC testing in the ambulance should consider the use of ultrasensitive troponin, randomization, and integration of troponin testing into an algorithm that crosses health care boundaries. Enhanced and more cost‐effective early ED discharge of the majority of patients with CP calling 911 is an unrealized opportunity.
Sources of Funding
Grant support was received from the Heart and Stroke Foundation of Canada. Additional in‐kind support was provided by Alberta Health Services, the University Hospital Foundation, and the Canadian VIGOUR Center. Alere Inc provided in‐kind support with test kits, training, and logistical support.
Disclosures
Koshy is an employee of Alere Inc. No other authors have relevant disclosures.
Supporting information
Appendix S1. PROACT‐4 Organization.
Acknowledgments
The authors are indebted to the patients enrolled in PROACT‐4 and grateful for the support from the Heart and Stroke Foundation of Canada, the University Hospital Foundation, the Mazankowski Alberta Heart Institute, Alberta Health Services, Alere Inc, and the dedicated work of the EMS personnel (listed in Appendix S1).
(J Am Heart Assoc. 2015;4:e002859 doi: 10.1161/JAHA.115.002859)
An accompanying Appendix S1 is available at http://jaha.ahajournals.org/content/4/12/e002859/suppl/DC1
Presented as a Late Breaking Clinical Trial at the American Heart Association Scientific Sessions November 7–11, 2015 in Orlando, FL.
References
- 1. Bhuiya FA, Pitts SR, McCaig LF. Emergency department visits for chest pain and abdominal pain: United States, 1999–2008. NCHS Data Brief. 2010;43:1–8. [PubMed] [Google Scholar]
- 2. Farkouh ME, Smars PA, Reeder GS, Zinsmeister AR, Evans RW, Meloy TD, Kopecky SL, Allen M, Allison TG, Gibbons RJ, Gabriel SE. A clinical trial of a chest‐pain observation unit for patients with unstable angina. Chest Pain Evaluation in the Emergency Room (CHEER) Investigators. N Engl J Med. 1998;339:1882–1888. [DOI] [PubMed] [Google Scholar]
- 3. Mehta RH, Eagle KA. Missed diagnoses of acute coronary syndromes in the emergency room–continuing challenges. N Engl J Med. 2000;342:1207–1210. [DOI] [PubMed] [Google Scholar]
- 4. Apple FS, Collinson PO; for the IFCC Task Force on Clinical Applications of Cardiac Biomarkers . Analytical characteristics of high‐sensitivity cardiac troponin assays. Clin Chem. 2011;58:54–61. [DOI] [PubMed] [Google Scholar]
- 5. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD; Joint ESC/ACCF/AHA/WHF Task Force for Universal Definition of Myocardial Infarction, Authors/Task Force Members Chairpersons, Biomarker Subcommittee , Katus HA, Apple FS, Lindahl B, Morrow DA; ECG Subcommittee , Clemmensen PM, Johanson P, Hod H; Imaging Subcommittee , Underwood R, Bax JJ, Bonow JJ, Pinto F, Gibbons RJ; Classification Subcommittee , Fox KA, Atar D, Newby LK, Galvani M, Hamm CW; Intervention Subcommittee , Uretsky BF, Steg PG, Wijns W, Bassand J‐P, Menasche P, Ravkilde J; Trials & Registries Subcommittee , Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez‐Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S; ESC Committee for Practice Guidelines (CPG) , Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Torbicki A, Vahanian A, Windecker S; Document Reviewers , Morais J, Aguiar C, Almahmeed W, Arnar DO, Barili F, Bloch KD, Bolger AF, Bøtker HE, Bozkurt B, Bugiardini R, Cannon C, de Lemos J, Eberli FR, Escobar E, Hlatky M, James S, Kern K, Moliterno DJ, Mueller C, Neskovic AN, Pieske BM, Schulman SP, Storey RF, Taubert KA, Vranckx P, Wagne DR. Third universal definition of myocardial infarction. [Internet]. J Am Coll Cardiol. 2012;60:1581–1598.22958960 [Google Scholar]
- 6. Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, Biedert S, Schaub N, Buerge C, Potocki M, Noveanu M, Breidthardt T, Twerenbold R, Winkler K, Bingisser R, Mueller C. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858–867. [DOI] [PubMed] [Google Scholar]
- 7. Than M, Cullen L, Aldous S, Parsonage WA, Reid CM, Greenslade J, Flaws D, Hammett CJ, Beam DM, Ardagh MW, Troughton R, Brown AFT, George P, Florkowski CM, Kline JA, Peacock WF, Maisel AS, Lim SH, Lamanna A, Richards AM. 2‐Hour accelerated diagnostic protocol to assess patients with chest pain symptoms using contemporary troponins as the only biomarker: the ADAPT trial. J Am Coll Cardiol. 2012;59:2091–2098. [DOI] [PubMed] [Google Scholar]
- 8. Sørensen JT, Terkelsen CJ, Steengaard C, Lassen JF, Trautner S, Christensen EF, Nielsen TT, Bøtker HE, Andersen HR, Thygesen K. Prehospital troponin T testing in the diagnosis and triage of patients with suspected acute myocardial infarction. Am J Cardiol. 2011;107:1–5. [DOI] [PubMed] [Google Scholar]
- 9. Roth A, Malov N, Golovner M, Sander J, Shapira I, Kaplinsky E, Laniado S. The, “SHAHAL” experience in Israel for improving diagnosis of acute coronary syndromes in the prehospital setting. Am J Cardiol. 2001;88:608–610. [DOI] [PubMed] [Google Scholar]
- 10. Leshem‐Rubinow E, Abramowitz Y, Malov N, Hadad M, Tamari M, Golovner M, Roth A. Prehospital cardiac markers in defining ambiguous chest pain. Arch Intern Med. 2011;171:2056–2057. [DOI] [PubMed] [Google Scholar]
- 11. Ezekowitz JA, Welsh RC, Gubbels C, Brass N, Chan M, Keeble W, Khadour F, Koshy TL, Knapp D, Sharma S, Sookram S, Tymchak W, Weiss D, Westerhout CM, Armstrong PW. Providing rapid out of hospital acute cardiovascular treatment 3 (PROACT‐3). Can J Cardiol. 2014;30:1208–1215. [DOI] [PubMed] [Google Scholar]
- 12. Triage Cardio2 Panel Monograph [Internet]. 2011. Available at: http://www.alere.ca/pdf/Triage-Cardio-Kit-EN-PI.pdf. Accessed September 3, 2013.
- 13. Sepehrvand N, Zheng Y, Armstrong PW, Welsh R, Goodman SG, Tymchak W, Khadour F, Chan M, Weiss D, Ezekowitz JA. Alignment of site versus adjudication committee‐based diagnosis with patient outcomes: insights from the Providing Rapid Out of Hospital Acute Cardiovascular Treatment 3 trial. Clin Trials. 2015; DOI: 10.1177/1740774515601437 [DOI] [PubMed] [Google Scholar]
- 14. De Luca G, Suryapranata H, Ottervanger JP, Antman EM. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation. 2004;109:1223–1225. [DOI] [PubMed] [Google Scholar]
- 15. Fox KAA, Dabbous OH, Goldberg RJ, Pieper KS, Eagle KA, Van de Werf F, Avezum A, Goodman SG, Flather MD, Anderson FA, Granger CB. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ. 2006;333:1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Validity of a risk‐prediction tool for hospital mortality: the Global Registry of Acute Coronary Events. Am Heart J. 2009;157:1097–1105. [DOI] [PubMed] [Google Scholar]
- 17. Goodacre SW, Bradburn M, Cross E, Collinson P, Gray A, Hall AS; RATPAC Research Team . The Randomised Assessment of Treatment using Panel Assay of Cardiac Markers (RATPAC) trial: a randomised controlled trial of point‐of‐care cardiac markers in the emergency department. Heart. 2011;97:190–196. [DOI] [PubMed] [Google Scholar]
- 18. Ryan RJ, Lindsell CJ, Hollander JE, O'Neil B, Jackson R, Schreiber D, Christenson R, Gibler WB. A multicenter randomized controlled trial comparing central laboratory and point‐of‐care cardiac marker testing strategies: the Disposition Impacted by Serial Point of Care Markers in Acute Coronary Syndromes (DISPO‐ACS) trial. Ann Emerg Med. 2009;53:321–328. [DOI] [PubMed] [Google Scholar]
- 19. Authors/Task Force Members , Roffi M, Patrono C, Collet J‐P, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST‐Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2015;ehv320. [Epub ahead of print]. [Google Scholar]
- 20. Reichlin T, Twerenbold R, Wildi K, Rubini Gimenez M, Bergsma N, Haaf P, Druey S, Puelacher C, Moehring B, Freese M, Stelzig C, Krivoshei L, Hillinger P, Jäger C, Herrmann T, Kreutzinger P, Radosavac M, Weidmann ZM, Pershyna K, Honegger U, Wagener M, Vuillomenet T, Campodarve I, Bingisser R, Miró O, Rentsch K, Bassetti S, Osswald S, Mueller C. Prospective validation of a 1‐hour algorithm to rule‐out and rule‐in acute myocardial infarction using a high‐sensitivity cardiac troponin T assay. Can Med Assoc J. 2015;187:E243–E252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. PROACT‐4 Organization.


