Figure 1.
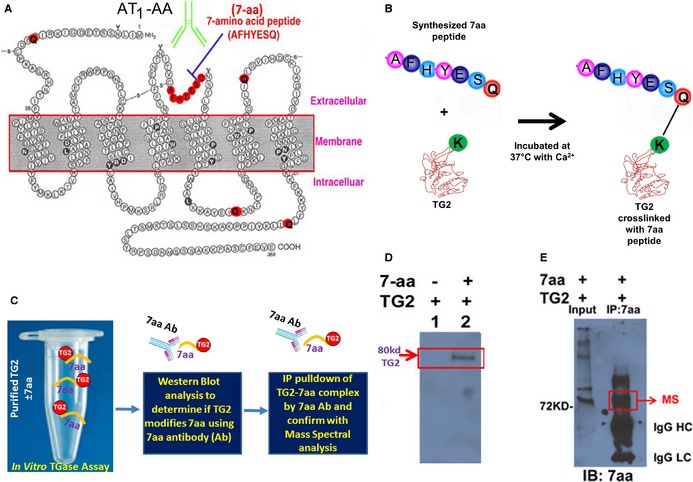
AT 1 receptor seven amino acid (7‐aa) epitope peptide is modified by TG2 in vitro. A, Two dimensional illustration of AT 1 receptor showing location of the 7‐aa epitope peptide and 5 glutamines (Q). B and C, Illustration of experimental strategy to determine whether TG2 modifies the 7‐aa epitope peptide in vitro. D, 7‐aa epitope peptide was covalently cross‐linked to TG2 in the in vitro assay. TG2 was incubated in the presence or absence of the 7‐aa epitope peptide (AFHYESQ). The reaction mix was subsequently fractionated by denaturing gel electrophoresis and transferred to a blot. Antibody specific for the 7‐aa epitope peptide recognized an 80‐kDa protein, the size expected for TG2, indicating that the 7‐aa peptide was covalently cross‐linked to TG2. E, Mass spectral characterization of TG2 cross‐linked with the 7‐aa epitope peptide. After the in vitro reaction, proteins covalently linked with 7‐aa epitope peptide in the reaction mix were immunoprecipitated with the antibody directed against this peptide and resolved in the denaturing gel electrophoresis. The 80‐kDa protein in the immunoprecipitation products was characterized as TG2 in mass spectral analysis. The presence of 7‐aa epitope in the immunoprecipitation products was also confirmed in Western blot using the antibody. AT receptor indicates angiotensin receptor 1; IgG HC, immunoglobulin G heavy chain; IgG LC, immunoglobulin G light chain; TG2, transglutaminase 2; MS, mass spectral analysis.
